Unit 3 Test Review
advertisement

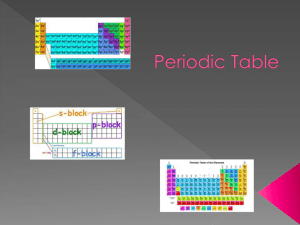
Name: __________________________________________ Chemistry -Unit 3 Periodic Test Review The periodic table and its trends 1) On the table to the right label the location for these groups – Halogens, Noble Gases, Alkaline Earth Metals, Alkali Metals, and Transition Metals 2) On the periodic table below circle the most electronegative element and put a square around the least electronegative element. 3) Shade the element with the highest ionization energy red and the element with the lowest blue. 1 5) On the table above - number the periods. 6) Shade period 4 in blue. 7) The rows are periods and the columns are _________. 8) The periodic table is organized according to increasing __________ ___________ 9) On the table to the left – use arrows to show the trend of increasing atomic size & radius. Circle the atom with the largest atomic size & radius. 10) Shade the metals in red, the nonmetals in blue, and metalloids in green. Identify the group by its’ description (halogens, alkali earth ____________________ 11) Have 1 valence electron ____________________ 12) Have 8 valence electrons ____________________ 13) Have 7 valence electrons ____________________ 14) Have 2 valence electrons ____________________ 15) The least reactive elements metals, noble gases, transition metals, and alkali metals) ____________________ 16) The most reactive nonmetals ____________________ 17) The most reactive metals ____________________ 18) Reacts explosively with oxygen and water ____________________ 19) Used to make coins &jewelry. Match to its correct definition (electronegativty and ionization energy) ____________________ 20) Energy required to remove an electron from an atom ____________________ 21) The ability of atom to attract electrons. 22) List 3 other elements that will have similar chemical properties to Nitrogen. Why do they have similar properties? 23) How many electrons does an atom of Oxygen have? _____ How many electrons does 0-2 have? _____ 24) Fluorine has a higher ionization energy than oxygen because fluorine has a larger ____________ charge (why fluorine holds on to its electrons so well) 2 Electron configuration Refer to the configuration to answer the following 4 questions: 1s2 2s2 2p6 3s2 3p6 4s2 25) Which element is this for? ____________________ 27) In which group #? _______ 26) In which period is this element found in?______ 27) How many electrons are in the 3rd energy level? ____ 28) Re-write the electron configuration of this element if it became an ion with a +2 charge. 29) What is an orbital? A ____________ shaped region of an atom where an ____________ is most likely to be found. 30) How many orbitals are in each sublevel? a. S = ___ b. P = ___ c. D = ___ d. F = ____ 31) How many electrons can fit into 1 orbital? _____ 32) Is the following electron configuration sketch correct? Explain. 3s 3p 4s 3d 33) On the table to the left – color and label these blocks: the s block (red) the d block (green) the p block (yellow) the f block (brown) 34) Identify the blocks where these groups are found – a. Halogens = ____ block b. Alkaline Earth Metals = _____ block c. Alkali Metals = ______ block d. Rare Earth Metals = ______ block e. The Noble Gases = _____ block f. The Transition Metals = _______ block 3 Photons and the Electromagnetic Spectrum 35) Use the known spectrum samples at the right to identify the unknowns below. 36) An electron that is closest to the nucleus possible (at the lowest energy level possible) is said to be in its ___________ _________________. 37) When an electron jumps up to a higher energy level and then falls back to its ground state a ___________ is released. 38) Please circle the answer that correctly completes the sentence. The amount of energy released by an electron jumping from the 4th energy level back to its ground state at the 3rd energy level will always release a photon with a different or variable/specific or quantized amount of energy. 39) The color spectra (types of colors produced) of a star can be used to identify the types of ______________ present in the star or chemical sample. 4