water and solutions - Nanomedicine Group
advertisement

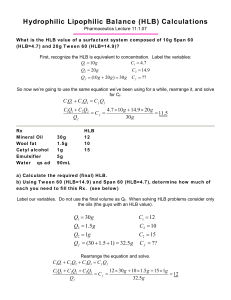
SURFACTANTS IN SOLUTION Amphiphilic Surfactants O O O + Na-O S O O O Aerosol OT Amphiphilic surfactants contain a non-polar portion and a polar portion. Classification of Surfactants O S - + O Na O Anionic Sodium dodecylsulfate (SDS) + N Br- Cationic Cetylpyridinium bromide O Zwitterionic O O OCH2CH2N(CH3)3+ P OO O Nonionic Dipalmitoylphosphatidylcholine (lecithin) O O O Polyoxyethylene(4) lauryl ether (Brij 30) O OH Surfactant Aggregates Unimers Normal micelles cylindrical spherical Inverted hexagonal phase Reverse micelles Bilayer lamella 4 nm Molecular Architecture Aerosol OT Sodium dodecylsulfate (SDS) Critical Micelle Concentration CMC 14 12 10 8 6 CMC 4 2 0 0 1 Surfactant concentration Below CMC only unimers are present Above CMC there are micelles in equilibrium with unimers Solution Properties 14 12 10 8 6 4 2 0 Concentration unimers CMC micelles 0 14 10 8 6 4 2 0 CMC 0 1 1/2 (Surfactant concentration) Osmotic pressure 10 8 6 4 2 0 Surfactant concentration1 Molar conductivity 1/R 12 14 12 CMC 0 14 Isc12 10 8 6 4 2 0 Surfactant concentration1 Light scattering CMC 0 Surfactant concentration1 Solubilization Spontaneous transfer of a compound insoluble in the bulk solvent into solution due to incorporation into the surfactant micelles Normal micelles non-polar compound amphiphilic compound Reverse micelles polar compound Solubility Effects Solubility of a poorly soluble compound increases as a result of solubilization in the micelles Solubility 14 12 10 8 6 CMC 4 2 0 0 1 Surfactant concentration HLB and Use of Surfactants Amphiphilic surfactants are characterized by the hydrophilic-lipophilic balance (HLB): a relative ratio of polar and non-polar groups in the surfactant HLB ca. 1 to 3.5: Antifoams HLB ca. 3.5 to 8: Water-in-Oil Emulsifiers HLB ca. 7 to 9: Wetting and spreading agents HLB ca. 8 to 16: Oil-in-Water Emulsifiers HLB ca. 13 to 16: Detergents HLB ca. 15 to 40: Solubilizers Required HLB HLB needed for emulsification of the oil phase. If there are several oil ingredients the required HLB is calculated as a sum of their respective required HLB multiplied by the fraction of each. Calculate the required HLB for the oil phase of the following o/w emulsion: cetyl alcohol 15 g., white wax 1g. Lanolin 2 g, emulsifier (q.s.), glycerin 5 g. water 100 g. Required HLB Cetyl alcohol White wax Lanolin Total required HLB (from reference) 15 x 15/18 12 x 1/18 10 x 2/18 Fraction 12.5 0.7 1.1 14.3 HLB of Surfactant Blend Surfactant blends are commonly used to obtain desired emulsifying properties. What is the HLB of the mixture of 40 % Span 60 (HLB = 4.7) and 60 % Tween 60 (HLB = 14.9)? HLB of mixture: 4.7 x 0.4 + 14.9 x 0.6 = 10.8 In what proportion should Span 80 (HLB = 4.3) and Tween 80 (HLB = 15.0) be mixed to obtain “required” HLB of 12.0? 4.3.(1-x) + 15.x = 12 x = 0.72 72 % Tween 80 and 28 % Span 80