Sumatriptan Actavis coated tablet ENG SmPC
advertisement

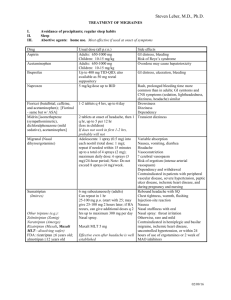
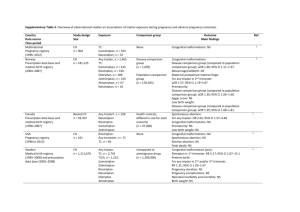
Läkemedelsverket 2013-07-12 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Sumatriptan Actavis 50 mg coated tablets Sumatriptan Actavis 100 mg coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains 50 mg or 100 mg sumatriptan (as sumatriptan succinate). Excipient with known effect: 50 mg: Lactose monohydrate and anhydrous lactose corresponding to 176 mg anhydrous lactose. 100 mg: Lactose monohydrate and anhydrous lactose corresponding to 352 mg anhydrous lactose. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Coated tablet (tablet). 50 mg: white, oval, biconvex tablets with scoring on both sides and on the edges, imprinted with "SN" on one side and "50" on the other. 100 mg: white, oval, biconvex tablets, imprinted with "SN" on one side and "100" on the other. 50 mg: the tablet can be divided into equal doses 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Acute treatment of migraine with or without aura. 4.2 Posology and method of administration Sumatriptan should not be used prophylactically. Sumatriptan is recommended as monotherapy for the acute treatment of migraine and should not be given concomitantly with ergotamine or derivatives of ergotamine (including methysergide) (see section 4.3). Sumatriptan should be taken as early as possible after migraine pain has appeared. However, sumatriptan is equally effective when administered at a later time during the attack. The following recommended dosages should not be exceeded. Posology Adults The recommended dose for adults is a single dose of 50 mg. For some patients, 100 mg may be necessary. Although the recommended oral dose of sumatriptan is 50 mg, it must be taken into account that the severity of migraine attacks varies both within and between patients. 1 If the patient does not respond to the first dose of sumatriptan, another dose should not be taken for the same attack. Sumatriptan may be used to treat subsequent attacks. If the patient has responded to the first dose, but the symptoms recur, a second dose may be given in the next 24 hours, provided that there is a minimum interval of 2 hours between the two doses. No more than 300 mg should be taken in any 24-hour period. Paediatric population The efficacy and safety of sumatriptan in children aged less than 10 years have not been established. No clinical data are available in this age group. The efficacy and safety of sumatriptan in children 10 to 17 years of age have not been demonstrated in the clinical trials performed in this age group. Therefore the use of Sumatriptan in children 10 to 17 years of age is not recommended (see section 5.1). Elderly There is limited experience of the use of sumatriptan in patients over the age of 65 years. The pharmacokinetics of the medicinal product in elderly patients has not been studied enough. The use of sumatriptan in patients over 65 years is not recommended until more clinical data are available. Hepatic insufficiency Patients with mild to moderate liver insufficiency: low doses of 25-50 mg should be considered for patients with mild to moderate liver impairment. Renal insufficiency See section 4.4. Method of administration The tablets should be swallowed whole with water. The tablets may be crushed and suspended in liquid. 4.3 Contraindications Hypersensitivity to the active substance, or to any of the excipients listed in section 6.1. A history of myocardial infarction or diagnosed coronary artery disease, Prinzmetal's angina/coronary artery spasm, peripheral vascular disease, or symptoms or signs of ischemic heart disease. A history of cerebrovascular accident (CVA) or transient ischemic attacks (TIA). Severe hepatic impairment. Moderate or severe hypertension and mild, uncontrolled hypertension. Concomitant use of ergotamine or ergotamine derivatives (including methysergide) or any triptan/5hydroxytryptamine1 (5-HT1) receptor agonist and sumatriptan is contraindicated (see section 4.5). Concomitant treatment with reversible (e.g. moclobemide) or irreversible (e.g. selegiline) monoamine oxidase inhibitors (MAOI's) and sumatriptan is contraindicated. Sumatriptan must not be used within 2 weeks of discontinuation of treatment with MAOI. 4.4 Special warnings and precautions for use 2 Sumatriptan Actavis tablets should only be used in cases where the diagnosis of migraine is definite. Sumatriptan Actavis is not indicated in hemiplegic, basilar or ophthalmoplegic migraine. As with other acute migraine therapies, serious neurological conditions should be excluded before treating a patient with newly diagnosed migraine or migraine patients who have atypical symptoms. It should be noted that migraineurs may be at increased risk of certain cerebrovascular events (e.g. CVA, TIA). After intake of sumatriptan, transient symptoms may occur, especially chest pain and tightness, which may be intense and radiate to the throat (see section 4.8). If there is any suspicion that these symptoms indicate ischaemic heart disease, treatment with sumatriptan should be discontinued and appropriate investigations carried out. Sumatriptan should be given with caution in patients with mild controlled hypertension, since transient increases in blood pressure and peripheral vascular resistance have been observed in a small proportion of patients (see section 4.3). Sumatriptan should not be administered to patients at risk of developing ischemic heart disease, including heavy smokers and heavy consumers of nicotine substitutes, until an evaluation excluding cardiovascular disease has been carried out (see section 4.3). This should be especially noted when prescribing to postmenopausal women and men over 40 years of age who have these risk factors. Such investigation will, however, not identify all patients with heart disease. In very rare cases, severe cardiovascular conditions have occurred even in patients without underlying heart disease. There have been rare post-marketing reports describing patients with serotonin syndrome (including altered mental status, autonomic instability and neuromuscular abnormalities) following the use of a selective serotonin reuptake inhibitor (SSRI) and sumatriptan. Serotonin syndrome has been reported following concomitant treatment with triptans and serotonin noradrenaline reuptake inhibitors (SNRIs). If concomitant treatment with sumatriptan and an SSRI/SNRI is clinically warranted, appropriate observation of the patient is advised (see section 4.5). Caution should be exercised when administering sumatriptan to patients with conditions which may affect absorption, metabolism or excretion of sumatriptan, e.g. hepatic or renal impairment. Sumatriptan should be given with caution to patients with a history of seizures or other risk factors, which reduce the seizure threshold, as seizures have been reported in association with sumatriptan (see section 4.8). Patients with known hypersensitivity to sulphonamides may react to sumatriptan with allergic reactions ranging from cutaneous hypersensitivity reactions to anaphylaxis. Evidence of cross allergy is limited. However, caution should be exercised when administering sumatriptan to these patients. With co-administration of triptans and herbal remedies containing St. John's Wort (Hypericum perforatum), the incidence of undesirable effects may increase. Prolonged use of any type of painkiller for headaches can make them worse. If this situation is experienced or suspected, medical advice should be obtained and treatment should be discontinued. The diagnosis of MOH (Medication overuse headache) should be suspected in patients who have frequent or daily headaches despite (or because of) the regular use of headache medications. The recommended dose of Sumatriptan Actavis should not be exceeded. 3 The tablet contains lactose monohydrate. Patients with any of the following rare hereditary disorders should not use this medicine: galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption. 4.5 Interaction with other medicinal products and other forms of interaction There is no evidence of interactions with propranolol, flunarizine, pizotifen or alcohol. Information is limited regarding interaction with medicinal products containing ergotamine or another triptan/5-HT1 receptor agonist. Co-administration is contraindicated as, theoretically, there is an increased risk of coronary artery spasm. The period of time that should elapse between the use of sumatriptan and ergotamine-containing preparations or another triptan/5-HT1 receptor agonist is not known. It depends on both the dose and the type of products used. The effects may be additive. At least 24 hours should elapse after the patient has taken a product containing ergotamine or another triptan/5-HT1 receptor agonist before sumatriptan is given. Conversely, it is advised to wait at least 6 hours following use of sumatriptan before administering an ergotamine-containing product and at least 24 hours before administering another triptan/5-HT1 receptor agonist (see section 4.3). Interactions may occur between sumatriptan and MAOI's and co-administration is contraindicated (see section 4.3). There have been rare post-marketing reports describing patients with serotonin syndrome (including altered mental status, autonomic instability and neuromuscular abnormalities) following the use of SSRIs and sumatriptan. Serotonin syndrome has also been reported following concomitant treatment with triptans and SNRIs (see section 4.4). There may also be a risk of serotonin syndrome if sumatriptan and lithium are given concomitantly. 4.6 Fertility, pregnancy and lactation Pregnancy Post-marketing data are available from over 1000 pregnancies in which exposure to sumatriptan occurred during the first trimester. Although there is insufficient information for definite conclusions, there is no indication of an increased risk of congenital defects. Experience of treatment with sumatriptan during the second and third trimester is limited. Animal studies give no indications of direct teratogenic effects or harmful effects to the peri- and postnatal development. However, survival in rabbit foetuses may be affected (see section 5.3). Pregnant women should only be treated with sumatriptan if the anticipated benefit to the mother outweighs the possible risk to the foetus. Breastfeeding Sumatriptan is excreted in breast milk after subcutaneous administration. To reduce the effect on the child, breast feeding should be avoided for 12 hours after treatment with sumatriptan, during which time any breast milk expressed should be discarded. 4.7 Effects on ability to drive and use machines No studies on the effects of sumatriptan on the ability to drive and use machines have been performed. Drowsiness may occur in a migraine patient due to the migraine attack or the treatment with sumatriptan. This may affect the ability to drive and use machines. 4.8 Undesirable effects 4 Undesirable effects are listed below according to organ system and frequency. Frequency is defined as: very common (≥1/10), common (≥1/100, <1/10), uncommon (≥1/1000, <1/100), rare (≥1/10,000, <1/1000), very rare (<1/10,000), not known (cannot be estimated from the available data). Some of the symptoms reported as undesirable effects may be associated symptoms of migraine. Immune system disorders Very rare: Hypersensitivity reactions varying from cutaneous hypersensitivity reactions (such as urticaria) to anaphylaxis. Nervous system disorders Common: Sensory disturbance including paraesthesia and hypoaesthesia, dizziness, drowsiness. Very rare: Seizures. Although some of these patients have a history of seizure or concurrent conditions predisposing to seizures. There are also reports in patients without any clearly predisposing factors. Nystagmus, scotoma, tremor, dystonia. Eye disorders Very rare: Flickering, diplopia, amblyopia, impaired vision. Loss of vision including reports of permanent sight reduction. However, the migraine attack itself may cause disturbances in vision. Cardiac disorders Very rare: Bradycardia, tachycardia, palpitations, cardiac arrhythmias, transient ischemic ECG changes, coronary artery vasospasm, angina or myocardial infarction (see section 4.3 and 4.4). Vascular disorders Common: Transient increase in blood pressure arising soon after treatment. Flushing. Very rare: Hypotension, Raynaud's phenomenon. Respiratory, thoracic and mediastinal disorders Common: Dyspnoea. Gastrointestinal disorders Common: Nausea and vomiting; however, relationship to sumatriptan has not been established. Very rare: Ischemic colitis. Not known: Diarrhoea. Musculoskeletal and connective tissue disorders Common: Feeling of heaviness (usually transient, may be intensive and of varying location including chest and throat). Myalgia. Very rare: Neck stiffness. Not known: Arthralgia. General disorders and administration site conditions Common: Pain, feeling of heat or cold, pressure or tightness (these symptoms are normally transient, and may be intensive and of varying location including chest and throat). Feelings of weakness and fatigue (both symptoms are normally mild to moderate in intensity and transient) Investigations Very rare: Minor disturbances in liver function tests. Psychiatric disorders Not known: Anxiety. Skin and subcutaneous tissue disorders Not known: Hyperhidrosis. 4.9 Overdose 5 Symptoms Patients have received up to 12 mg sumatriptan as a subcutaneous single injection with no significant undesirable effects. Occasional doses of sumatriptan of more than 16 mg subcutaneously and 400 mg orally, have caused no other side effects than those given in section 4.8. Treatment On overdose, the patient should be monitored for at least ten hours and given suitable symptomatic treatment. There is no documentation on the effect of haemodialysis or peritoneal dialysis on plasma concentrations of sumatriptan. 5. PHARMACOLOGICAL PROPERTIES 5.1 Pharmacodynamic properties Pharmacotherapeutic group: Antimigraine preparations, Selective serotonin (5-HT1) agonists ATC code: N02CC01 Sumatriptan is a selective agonist of vascular 5-hydroxytryptamine-1 receptors with no effect on other 5HT receptors. This type of receptor is mainly found in cranial blood vessels. In animals, sumatriptan selectively constricts the carotid arterial circulation which supplies the extra- and intracranial tissues such as the meninges. Dilatation of these blood vessels is thought to be the underlying cause of migraine in humans. Animal studies also suggest that sumatriptan inhibits trigeminal nerve activity. Both of these mechanisms (cranial artery constriction and inhibition of trigeminal nerve activity) may contribute to the effect of sumatriptan in humans. The clinical response begins about 30 minutes after an oral dose of 100 mg. Paediatric population A number of placebo-controlled clinical studies assessed the safety and efficacy of oral sumatriptan in approximately 800 children and adolescent migraineurs aged 10 to 17 years. These studies failed to demonstrate relevant differences in headache relief at 2 hours between placebo and any sumatriptan dose. The undesirable effects profile of oral sumatriptan in adolescents aged 10-17 years was similar to that reported from studies in the adult population. 5.2 Pharmacokinetic properties Following oral administration, sumatriptan is rapidly absorbed and 70% of the maximum plasma concentration is reached within 45 minutes. After an oral dose of 100 mg, the mean maximum plasma concentration is 54 ng/ml. Mean oral bioavailability is 14%, partly due to presystemic metabolism and partly due to incomplete absorption. The elimination phase half-life is approximately 2 hours. Plasma protein binding is low (14-21%) and mean volume of distribution is 170 litres. Mean total plasma clearance is approximately 1160 ml/min and the mean renal plasma clearance is approximately 260 ml/min. Extra-renal clearance accounts for about 80% of the total clearance, indicating that sumatriptan is mainly eliminated through metabolism. The major metabolite, the indole acetic acid analogue of sumatriptan, is mainly excreted in the urine, as a free acid and the glucuronide conjugate. The metabolite has no known 5HT1 or 5HT2 activity. The other metabolites have not been identified. The pharmacokinetics of oral sumatriptan do not appear to be significantly affected by migraine attacks. 5.3 Preclinical safety data 6 In a rat fertility study with doses well above the maximum doses used in humans, a reduction in successful inseminations was seen. In rabbits, embryolethality without marked teratogenic defects was seen. The relevance for humans of these findings is unknown. Sumatriptan was devoid of genotoxic and carcinogenic activity in in-vitro systems and animal studies. 6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Tablet core: Lactose monohydrate Croscarmellose sodium Lactose, anhydrous Cellulose, microcrystalline Magnesium stearate Tablet coating: Lactose monohydrate Mannitol Titanium dioxide (E 171) Talc Triacetin 6.2 Incompatibilities Not applicable. 6.3 Shelf life 3 years 6.4 Special precautions for storage This medicinal product does not require any special storage conditions. 6.5 Nature and contents of container Blister packs of PVC/Aluminium or PVC/PVDC/Aluminium: 2, 3, 4, 6, 12, 18 and 24 tablets HDPE tablet containers with tamper evident LDPE lids: 2, 3, 4, 6, 12, 18 and 24 tablets Not all pack sizes may be marketed. 6.6 Special precautions for disposal No special requirements. 7. MARKETING AUTHORISATION HOLDER <[To be completed nationally]> 8. MARKETING AUTHORISATION NUMBER(S) 7 <[To be completed nationally]> 9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION 29-05-2007 10. DATE OF REVISION OF THE TEXT 2013-07-12 8