RNANotes - McMaster Physics and Astronomy
advertisement
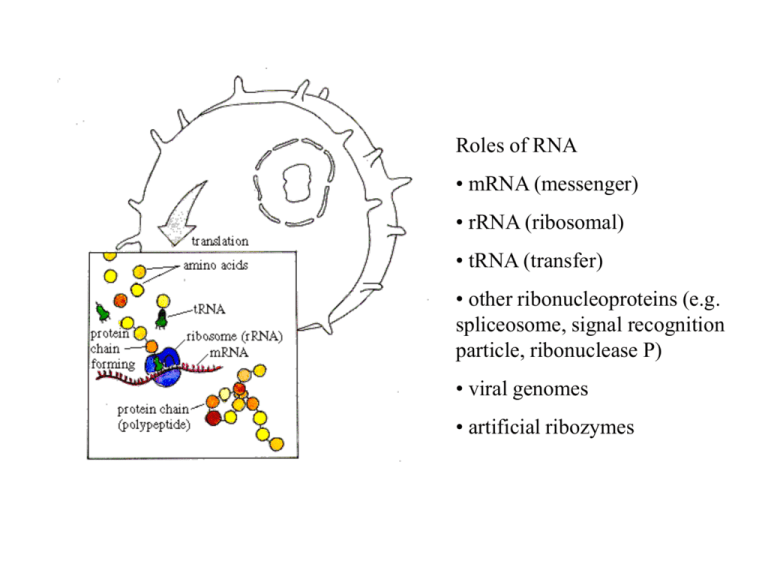
Roles of RNA • mRNA (messenger) • rRNA (ribosomal) • tRNA (transfer) • other ribonucleoproteins (e.g. spliceosome, signal recognition particle, ribonuclease P) • viral genomes • artificial ribozymes Typical transfer RNA structure Thermodynamics parameters are measured on real molecules. G Helix formation = hydrogen bonds + stacking U U C } DG = -2.1 kcal/mol A } DG = -1.2 kcal/mol A U loop DG = + 4.5 kcal/mol C Entropic penalty for loop formation. C U Multi-branched loop Sum up contributions of helices and loops over the whole structure. Bulges Internal loops Hairpin loop Pairs i-j and k-l are compatible if (a) i < j < k < l , or (b) i < k < l < j . (c) is called a pseudoknot: i < k < j < l . Usually not counted as secondary structure. (b) (a) (c) k k i k l l l j i i j j Bracket notation is used to represent structure: a: ((((....))))..((((....)))) b: ((.((((....)))).)) Basic problem: Want an algorithm that considers every allowed secondary structure for a given sequence and finds the lowest energy state. Simplest case: find structure which maximizes number of base pairs. Let ij = -1 if bases can pair and + if not. Ignore loop contributions. E(i,j) = energy of min energy structure for chain segment from i to j. We want E(1,N). or = i j i j-1 j i k j E (i, j 1) E (i, j ) min min E (i, k 1) E (k 1, j 1) kj i k j 4 Algorithms that work by recursion relations like this are called dynamic programming. The algorithm is O(N3) although the number of structures increases exponentially with N. Also need to do backtracking to work out the minimum energy structure: Set B(i,j) = k if j is paired with k, or 0 if unpaired. Partition Function Algorithm (for simplest energy rules) or = i j i j-1 j i k j 4 Z ij Z i , j 1 Z i ,k 1Z k 1, j 1akj k i where akj exp( kj / kT ) Real Energy Rules : Need to consider many special cases. What type of loop are you closing? Algorithm is more complex but still is O(N3). j Equilibrium probability that base i is paired with j Equilibrium probability that base i is unpaired pij aij Z i 1, j 1 Z ijends Z1, N 1 i j N pi ,0 1 pij j 1 Example of pairing probabilities taken from Vienna package web-site N Is folding kinetics important? RNA folding kinetics involves reorganisation of secondary structure i A C B D iii I ii B B C D E F H D G Native structures may not be global minimum free energy states. Morgan & Higgs (1996) J. Chem. Phys. Energy Landscapes in RNA Folding Morgan & Higgs (1998) Quantity Fitting Function Groundstate energy Parameters E C1 N C1 = 2.9 (0.2) = -0.368 (0.001) Total number of states ln C2 N C2 = -5.6 (0.4) = 0.533 (0.001) Number of groundstates ln C3 N C3 = 1.75 (0.2) = 0.068 (0.001) Groundstates are degenerate in this model because energies are integers. Generate many random groundstates. How far apart are these groundstates? How high are the barriers between groundstates? We found Frozen pairs (present in every groundstate) This figure shows the frozen pairs only. The molecule is divided into independent unfrozen loops. Define Neff as the length of the longest loop. Two groundstates for the same sequence Minimum Free Energy Prediction Deterministic. Always gets MFE structure for a given set of energy rules. If MFE structure is not the same as biological structure, this could be because (i) energy rules are inaccurate or insufficient (ii) kinetics is important and molecule is trapped in metastable state. Monte Carlo simulations of folding kinetics. Store a current structure. Estimate rates of removal of existing helices and rates of addition of other compatible helices. Choose one helix to be added or removed with probability proportional to its rate. Repeat this many times. Can simulate structure formation from an unfolded state. Q is a bacteriophage RNA virus with approx 4000 nucleotides Viral RNA has complex secondary structure. The replicase gene codes for the replicase protein. This is an RNA-dependent RNA polymerase. Synthesizes complementary strand. Viral replication needs two steps: plus to minus to plus. In vitro RNA evolution in the Q system c Begin with Replicase + nucleotides + viral RNA c Replicase + nucleotides only Transfer small quantity to each successive tube c c sequence RNA after many transfers Barrier heights between alternative groundstates Observation: Mean barrier height between groundstates scales as <h> ~ Neff0.5 Neff ~ 0.3 N Therefore barriers become significant for large enough sequences. An example where kinetics is important to control biological function: the 5’ region of the MS2 phage. 3500 130 Maturation protein Time to formation of the 5’ structure influences expression of the maturation protein more than the stability of this structure. Average prob. SD free Simulations compare with experiments on mutant sequences. CC3435AA 0.2 0.1 WT & U32C SA 0.0 0.0 2.0 4.0 Time (s) 6.0 8.0 RNA in comparison to Proteins Both have well defined 3d structures RNA folding problem is easier because secondary structure separates from tertiary structure more easily - But it is still a complex problem. RNA model has real parameters therefore you can say something about real molecules. RNA folding algorithm is simple enough to be able to do statistical physics. (cf. 27-mer lattice protein models). Part of sequence alignment of Mitochondrial Small Sub-Unit rRNA Full gene is length ~950 11 Primate species with mouse as outgroup Mouse Lemur Tarsier SakiMonkey Marmoset Baboon Gibbon Orangutan Gorilla PygmyChimp Chimp Human : : : : : : : : : : : : * 20 * 40 * 60 * CUCACCAUCUCUUGCUAAUUCAGCCUAUAUACCGCCAUCUUCAGCAAACCCUAAAAAGG-UAUUAAAGUAAGCAAAAGA CUCACCACUUCUUGCUAAUUCAACUUAUAUACCGCCAUCCCCAGCAAACCCUAUUAAGGCCC-CAAAGUAAGCAAAAAC CUUACCACCUCUUGCUAAUUCAGUCUAUAUACCGCCAUCUUCAGCAAACCCUAAUAAAGGUUUUAAAGUAAGCACAAGU CUUACCACCUCUUGCC-AU-CAGCCUGUAUACCGCCAUCUUCAGCAAACUCUA-UAAUGACAGUAAAGUAAGCACAAGU CUCACCACGUCUAGCC-AU-CAGCCUGUAUACCGCCAUCUUCAGCAAACUCCU-UAAUGAUUGUAAAGUAAGCAGAAGU CCCACCCUCUCUUGCU----UAGUCUAUAUACCGCCAUCUUCAGCAAACCCUGAUGAAGGCUACGAAGUGAGCGCAAAU CUCACCAUCUCUUGCU----CAGCCUAUAUACCGCCAUCUUCAGCAAACCCUGACAAAGGCUAUAAAGUAAGCACAAAC CUCACCACCCCUUGCU----CAGCCUAUAUACCGCCAUCUUCAGCAAACCCUGAUGAAGGCCACGAAGUAAGCGCAAAC CUCACCACCUCUUGCU----CAGCCUAUAUACCGCCAUCUUCAGCAAACCCUGACGAAGGCCACAAAGUAAGCACAAGU CUCACCGCCUCUUGCU----CAGCCUAUAUACCGCCAUCUUCAGCAAACCCUGAUGAAGGUUACAAAGUAAGCGCAAGU CUCACCGCCUCUUGCU----CAGCCUAUAUACCGCCAUCUUCAGCAAACCCUGAUGAAGGUUACAAAGUAAGCGCAAGU CUCACCACCUCUUGCU----CAGCCUAUAUACCGCCAUCUUCAGCAAACCCUGAUGAAGGCUACAAAGUAAGCGCAAGU CucACC cuCUuGCu cAgccUaUAUACCGCCAUCuuCAGCAAACcCu A G aAAGUaAGC AA : : : : : : : : : : : : 78 78 79 76 76 75 75 75 75 75 75 75 100 100 96 100 100 100 99 63 72 67 91 99 100 100 100 Murphy et al. Nature (2001) 100 100 66 77 100 100 100 96 93 100 100 100 100 100 < 50 < 50 63 92 100 90 100 100 74 99 99 52 70 100 100 96 97 100 100 97 98 uses 15 nuclear plus 3 mitochondrial proteins 100 100 100 100 100 90 100 70 100 100 100 90 100 53 98 88 < 50 < 50 < 50 55 65 64 79 100 100 100 72 100 87 97 87 100 97 84 71 83 99 52 95 99 95 100 100 100 85 62 < 50 93 64 85 < 50 < 50 72 60 < 50 < 50 100 100 100 100 100 100 100 67 100 100 < 50 100 < 50 98 80 99 100 100 100 100 100 100 100 77 99 100 100 100 100 100 100 94 < 50 99 97 100 63 100 < 50 79 100 < 50 < 50 50 < 50 < 50 100 100 Megaptera * Cetacea Tursiops Hippopotamus * Tragelaphus Okapia Sus Lama * Ceratotherium * Tapirus Equus * Felis * Leopardus Panthera Canis * Ursus Manis * Artibeus * Microchiroptera Nycteris Pteropus * Megachiroptera Rousettus Erinaceus * Sorex * Asioscalops Condylura * Cetartiodactyla Perissodactyla IV Carnivora Pholidota Chiroptera 'Eulipotyphla' Cavia * Hydrochaerus Agouti CavioHystricoErethizon morpha gnathi Myocastor Dinomys Hystrix Heterocephalus * Rodentia Mus * Rattus Cricetus Pedetes Castor Dipodomys Tamias * Muscardinus Sylvilagus * Lagomorpha Ochotona * Hylobates Homo * Macaca * Anthropoidea Primates Ateles * Callimico Cynocephalus */ ** Dermoptera ** Lemur * Lemuriformes Primates Tarsius * Tarsiiformes Tupaia * Scandentia Choloepus did. * Choloepus hof. Tamandua Xenarthra Myrmecophaga Euphractus * Chaetophractus Trichechus * Sirenia Tethytheria Loxodonta * Proboscidea Procavia * Hyracoidea Echinops * Tenrecidae Orycteropus * Tubulidentata Macroscelides Macroscelidea Elephantulus * Didelphis * Marsupialia Macropus * G l i r e s III II P a e n u n g u l a t a A f r o t h e r i a I Afrotheria / Laurasiatheria Striking examples of convergent evolution Cao et al. (2000) Gene uses 12 mitochondrial proteins RNA pairs model (GR7) 53 complete Mammalian mitochondrial genomes Complete set of rRNAs + tRNAs from = 973 pairs. 100 100 86 100 97 100 100 Jow et al. (2002) MCMC searches the rugged landscape in tree space using the Metropolis algorithm. Obtains a set of possible trees weighted according to their likelihood. 1. Rate parameter changes = continuous 2. Branch length changes = continuous E A 3. Topology changes = discrete D 2 E C A A E D 1 4 B Nearest-neighbour interchange D C Long-range move E B C C B D 3 B A Models of Sequence Evolution rij is the rate of substitution from state i to state j States label bases A,C,G & T Pij(t) = probability of being in state j at time t given that ancestor was in state i at time 0. dPij dt Pik rkj k i t j The HKY model describes rate of evolution of single sites to A from A G C T * G C C * T T T C * G A C T A A * G G The frequencies of the four bases are A , G , C , T . is the transition-transversion rate parameter * means minus the sum of elements on the row Compensatory Substitutions Two sides of the acceptor stem from a tRNA are shown. Due to structure conservation alignment is possible in widely different species. Bacillus subtilis Escherichia coli Saccharomyces cerevisiae Drosophila melanogaster Homo sapiens 1234567 ((((((( 7654321 ))))))) GGCUCGG GCCCGGA GCGGAUU GCCGAAA GCCGAAA CCGAGCC UCCGGGC AAUUCGC UUUCGGC UUUCGGC Model 7A is a General Reversible 7-state Model 7 frequencies i + 21 rate parameters ij - 2 constraints = 26 free parameters 1 2 3 4 5 6 7 AU GU GC UA UG CG MM 5 15 5 25 5 35 5 45 6 16 6 26 6 36 6 46 6 56 7 17 7 27 7 37 7 47 7 57 7 67 1 AU * 2 12 2 GU * 3 GC 4 UA 5 UG 6 CG 7 MM 1 12 1 13 1 14 1 15 1 16 1 17 2 23 2 24 2 25 2 26 2 27 3 13 4 14 3 23 4 24 * 4 34 3 34 * 3 35 4 45 3 36 4 46 3 37 4 47 * 5 56 * 5 57 6 67 * Probability of remaining in same state Pii SSU rRNA sequences from Eubacteria Probability Pij of changes from CG to other pairs SSU rRNA from Eubacteria What is going on? AU GU UA fast fast UG slow GC CG Selection against GU and UG is weaker than against mismatches. Double transitions are faster than double transversions. Double transitions are faster than single transitions to GU and UG states. This is explained by the theory of compensatory substitutions. Analysis of RNA sequence databases tRNA mitoch. tRNA general tRNA archaea Rnase P SSU rRNA G+C average G+C helical regions 0.339 0.448 0.532 0.681 0.636 0.829 0.594 0.730 0.545 0.674 Frequencies 0.266 0.121 0.257 0.233 0.046 0.030 0.046 0.372 0.260 0.128 0.142 0.043 0.025 0.030 0.473 0.320 0.057 0.077 0.031 0.020 0.022 0.385 0.296 0.117 0.104 0.050 0.022 0.026 0.352 0.298 0.122 0.173 0.020 0.021 0.014 Number of sequences 884 754 64 84 455 Number of pairs 21 21 21 80 296 GC CG AU UA GU UG MM Selection for thermodynamically stable structures Higgs (2000) Quart. Rev. Biophysics Analysis of RNA Substitution Rates tRNA mitoch. tRNA general tRNA archaea Rnase P SSU rRNA 0.67 0.84 0.86 0.77 2.44 3.32 2.32 0.49 0.83 1.46 1.24 1.96 5.01 0.99 0.45 0.89 4.01 1.78 1.85 3.00 0.86 0.65 0.60 1.46 1.09 1.72 2.84 5.24 0.55 0.66 1.40 0.93 3.92 4.36 7.84 Double transitions / Double transversions 4.7 1.7 2.3 3.1 2.1 Double transitions / Transitions to GU or UG 1.6 2.0 8.9 3.6 2.8 Mutabilities GC CG AU UA GU UG MM Thermodynamic properties influence Evolutionary properties



