Lecture 11: Signalling for Life/Death
advertisement
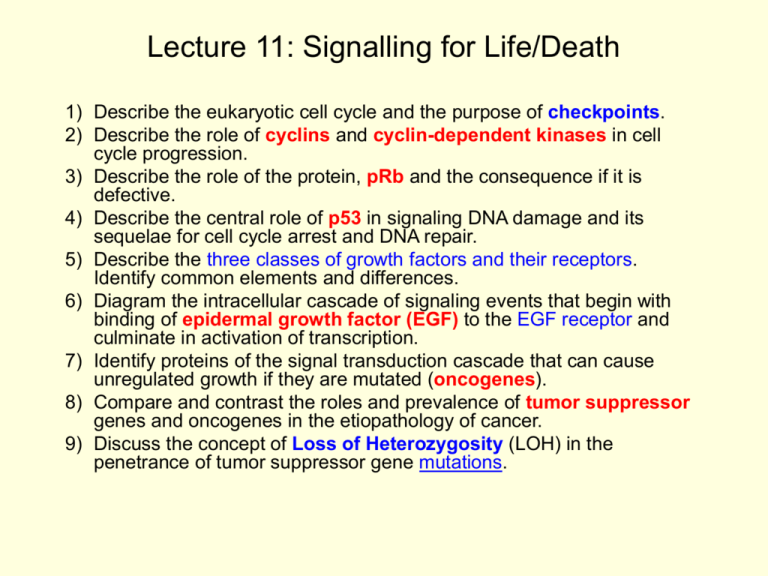
Lecture 11: Signalling for Life/Death 1) Describe the eukaryotic cell cycle and the purpose of checkpoints. 2) Describe the role of cyclins and cyclin-dependent kinases in cell cycle progression. 3) Describe the role of the protein, pRb and the consequence if it is defective. 4) Describe the central role of p53 in signaling DNA damage and its sequelae for cell cycle arrest and DNA repair. 5) Describe the three classes of growth factors and their receptors. Identify common elements and differences. 6) Diagram the intracellular cascade of signaling events that begin with binding of epidermal growth factor (EGF) to the EGF receptor and culminate in activation of transcription. 7) Identify proteins of the signal transduction cascade that can cause unregulated growth if they are mutated (oncogenes). 8) Compare and contrast the roles and prevalence of tumor suppressor genes and oncogenes in the etiopathology of cancer. 9) Discuss the concept of Loss of Heterozygosity (LOH) in the penetrance of tumor suppressor gene mutations. 1) Describe the eukaryotic cell cycle and the purpose of checkpoints. DNA nucleus cell Two Diploid Daughter Cells Diploid G1 Phase (M) Mitosis G2/M transition G2 Phase S Phase DNA Replication Tetraploid R point (starvation) START G1/S transition Most physiological growth arrest occurs here, as most cells in the body are 2n. 2) Describe the role of cyclins (A, D and E) and cyclindependent kinases (cdc2, CDK2 and CDK4) in cell cycle progression. CDK4/cyclin D/PCNA needed for R transit R point cdc2 needed for G2/M transit G1 phase Start PCNA CDK2/cyclins E and A are needed for G1/S transit PCNA = proliferating cell nuclear antigen 2) Describe the role of the retinoblastoma protein, pRb and the consequence if it is defective. pRb-Phos pRb-E2F complex active E2F CDK4 action G1 phase Start Genes PCNA If Rb is defective (loss of function), E2F will be unregulated and retinoblastoma will occur. Rb is a tumor suppressor. 2) Describe the central role of p53 in signaling DNA damage and its sequelae for cell cycle arrest and DNA repair. ATM 2 UbiquitinMediated Degradation 0 1 DNA damage p53 DNA repair 4b GADD-45 3 p21WAF 4a G1/S Arrest G1 phase PCNA If p53 is defective (loss of function), the cell cycle can proceed in spite of DNA damage, and mutations will accumulate. GADD = growth arrest & DNA damage inducible 5) Describe the three classes of growth factors and their receptors. Identify common elements and differences. I. Epidermal growth factor: EGF, FGF, NGF (FGF= fibroblast growth factor; NGF=nerve growth factor) II. III. Insulin like growth factor: Insulin, IGF-1, IGF-2 A. Platelet-derived growth factor: [no other examples] Common: • Receptors on cell surface. • Receptors active as dimers • Receptors have intracellular tyrosine kinase activity • Phosphorylated receptors attract SH2 proteins Differences: •Type II (IGF and insulin) receptors are dimers all the time •Ligands are different 6) Diagram the intracellular cascade of signaling events that begin with binding of epidermal growth factor (EGF) to the EGF receptor and culminate in activation of transcription. 1. EGF 2. EGF-R 3. Tyrosine kinase (receptor/nonreceptor) 4. 5. 6. 7. 8. 9. SH2 (SHC) Grb-2 SOS Ras (G protein) Raf (S/T kinase) MAP kinase cascade 10. Nuclear transcription factors cTK (abl) GRB -2 SOS ras raf MAP kinase “cascade” Phosphorylation of transcription factors Induction of immediate early genes (START) 6) Identify proteins of the signal transduction cascade that can cause unregulated growth if they are mutated (gain of function) (oncogenes). 1. Growth factors (EGF, FGF, PDGF) 2. Growth factor-R (EGF-R, NGF-R) 3. non-receptor tyrosine kinase (src, abl*) 4. G protein (Ras) 5. S/T kinase (Raf) 6. Nuclear transcription factors (myc) *abl can be inhibited by Gleevek. cTK (abl) GRB -2 SOS ras raf MAP kinase “cascade” Phosphorylation of transcription factors Induction of immediate early genes (START) 8) Compare and contrast the roles and prevalence of tumor suppressor genes and oncogenes in the etiopathology of cancer. • Proto-oncogenes genes are normal cellular proteins involved in positive regulation of proliferation. Unregulated growth occurs from: gain-of-function mutation of proto-oncogene resulting in oncogene. • Tumor suppressors are normal cellular proteins that are involved in negative regulation of proliferation. Unregulated growth occurs from a loss-of-function mutation of the tumor suppressor, and usually requires loss of both alleles. 9) Discuss the concept of Loss of Heterozygosity (LOH) in the penetrance of tumor suppressor genes. • Loss-of-function mutations are more common than are gain of function, yet both alleles must be inactivated. Hence, a major mechanism for loss of tumor suppression is loss of heterozygosity. • The most common mutations in cancers are: ras oncogene [gain or loss of function?] p53 tumor suppressor gene [what is consequence?] (ras and p53 are each mutated in >60% of all cancers).







