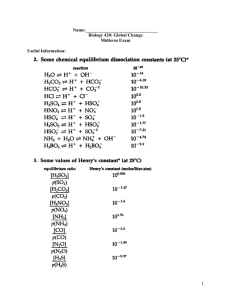
ppt file for this lecture
advertisement

The Sea Around Us Lecture 4: 21 January Water is The Wonder Substance: Physical & Chemical Properties Water Cycle Jump! Drown with Me Porcupine Tree. VV Brown, Shark In The Water Thanks to Rachel B., Zach R. Ocean Breathes Salty Modest Mouse • Lecture Review Questions: • On-line Assignment 2 is due tonight by 11pm • Homework 1 is available on Angel • Cell Phone Recycling: Benefit Relay for Life and The Ocean! • Book pics? (Angel dropbox) • Questions (Angel) Read about The Sea Around Us • Lecture notes and slides on the course web site • Your book The WONDER SUBSTANCE: Physical & Chemical Properties • Ice is less dense than water. • At 0°C, density of water is 1 gm/cc; Ice is 0.917 gm/cc • Ice has an open hexagonal structure Water molecular structure ice is about IceDensity molecularofstructure 91% of liquid water Density of Water • Fresh water reaches maximum density at 3.98 °C • Density= 1,000 kg/m3 (1kg/liter) • Density decreases as water is heated above 4°C • At 20 °C, density of pure H2O is 998.23 kg/m3 Water: The Wonder Substance Low viscosity •rapid flow to equalize pressure differences High surface tension •allows wind energy to be transmitted to sea surface •allows cells to hold shape --and life to form •controls the behavior of water drops High heat capacity •cools/warms slowly relative to land •aids in heat retention & transport •minimizes extremes in temperature •helps to maintain uniform body temps High latent heat of evaporation •very important in heat/water transfer in atmosphere Latent Heat and Changes of State Latent heat of fusion (or melting) • Heat to form or melt ice (liquid to solid phase) • 333 kJ/kg (80 calories/gram) Latent heat of vaporization (or precipitation) • Heat to vaporize (boil) a liquid or condense liquid from a gas phase • 2260 kJ/kg (540 calories/gram) Evaporation of water from the surface can occur at any temperature. However, it takes more energy to evaporate at low T than to boil off vapor once water reaches 100°C Heat capacity and phase changes: ice (solid) water (liquid) vapor or steam (gas) Latent Heat Heat needed to change phase (from solid to liquid, liquid to gas, liquid to solid, etc.) Heat required to change the temperature (by 1 °) of a given mass Let’s measure Heat Capacity Temperature (°C) Heat Capacity Rock & Soil 30 20 Liquid water Pepsi 10 10 50 Heat input (J/kg or cal/gram) Heat required to change the temperature (by 1 °) of a given mass Let’s measure Heat Capacity Temperature (°C) Heat Capacity Rock & Soil 30 20 Liquid water 10 10 50 Heat input (J/kg or cal/gram) Heat required to change the temperature (by 1 °) of a given mass Let’s measure Heat Capacity Temperature (°C) Heat Capacity Rock & Soil 30 20 Liquid water 10 10 50 Heat input (J/kg or cal/gram) Heat required to change the temperature (by 1 °) of a given mass Let’s measure Heat Capacity Temperature (°C) Heat Capacity Rock & Soil 30 20 Liquid water 10 10 50 Heat input (J/kg or cal/gram) Heat required to change the temperature (by 1 °) of a given mass Let’s measure Heat Capacity Temperature (°C) Heat Capacity Rock & Soil 30 20 Liquid water 10 10 50 Heat input (J/kg or cal/gram) Heat required to change the temperature (by 1 °) of a given mass The green line is longer than the yellow line! If we add the same amount of heat to two materials, the one with lower heat capacity will warm up more! Let’s measure Heat Capacity Temperature (°C) Heat Capacity Rock & Soil 30 20 Liquid water 10 10 50 Heat input (J/kg or cal/gram) Today is the first in-class iClicker exercise for credit A) Full credit if you answer 60% or more of the questions B) Bonus points if you get the correct answer for 80% of more of the questions C) If there are 10 questions and you answer at least 6 of them you’ll get full credit (100%) D) If there are 10 questions and you answer at least 8 of them correctly you’ll get a 5% bonus (105%) E) All of the above (this is the correct answer, choose E!) Heat capacity and phase changes: ice (solid) 150 water (liquid) vapor or steam (gas) Vapor 100 vapor+ liquid Liquid water 50 Latent Heat Heat needed to change phase (from solid to liquid, liquid to gas, liquid to solid, etc.) Latent heat of vaporization or condensation 540cal/gm Ice + liquid 0 -50 Ice Latent heat of fusion or melting 80cal/gm -100 0 200 400 600 Heat input (cal/gram) 800 Why the Sea is Salty Seawater is essentially an NaCl solution Average seawater salinity is 35 ppt (35 g/kg), but it varies from place to place 30 ppt Surface water salinity 37 ppt Why the Sea is Salty Why the Sea is Salty And over the eons of time, the sea has grown ever more bitter with the salt of the continents Ocean Salinity Was the Chemistry of the Ancient Oceans the Same as Today? 35 0/00 ? Time (billions of yrs) Surface water salinity Was the Chemistry of the Ancient Oceans the Same as Today? Ocean Salinity 35 0/00 Time (billions of yrs) Surface water salinity Note the attraction of oppositely charged ends of water molecules for one another Seawater is essentially an NaCl solution (saltwater) Cl-, Na+, S04-2, Mg+2, Ca+2, K+ >99% of salt in sea water HC03-2, Br-, Sr-2, B+2, F- (with these, 99.99%) http://www.webelements.com/ Seawater is essentially an NaCl solution Average seawater salinity is 35 ppt or 35 g/kg. Relative abundance of ions in seawater, in rank order: Cl, Na, SO4, Mg, Ca, K (these make up >99% of the salt in seawater) HCO3, Br, Sr, B, F (with these >99.99% of the salt in seawater) All other elements occur at very low concentrations (ppm to ppb: 10-6 to 10-9) Charges must balance, therefore: Charge associated with cations: Na+, Mg+2, Ca+2, K+ Must equal charge associated with anions: Cl-, SO4-2 Major ions in seawater keep “constant proportion,” regardless of salinity • Except near river outlets (near coastal regions) • Salinity (o/oo) ~1.81 x Chlorinity (o/oo) And in soils But rivers are not the only important input Ocean Chemistry is influenced by Erosion and Weathering of the land Rivers vs. Other Sources •Difference in chemical compositions between rivers and ocean --reflects sedimentation (precipitation) processes --other inputs/exchanges, such as basalt-seawater reactions at midocean ridges For example, exchange of Magnesium (Mg) in seawater for Ca in ocean crust supplies excess Calcium Oceans: Chemical Inputs -rivers (weathering) -volcanic gases: HCl, SO2, CO2 -interaction of seawater with seafloor, e.g., hot basalt associated with Hydrothermal Circulation, this is a source of Ca and K Note: A volume of water equal to the entire ocean is circulated through seafloor material (crust) ~ every 10 m.y. Ocean Chemistry is influenced by: A. water interacting with rocks (Earth’s crust) at the mid-ocean ridges B. Evaporation of seawater C. River water D. Erosion and weathering of the land E. All of the above. Why the Sea is Salty Seawater is essentially an NaCl solution Average seawater salinity is 35 ppt (35 g/kg), but it varies from place to place 30 ppt Surface water salinity 37 ppt Can we explain ocean chemistry using the inputs of rivers alone? atmosphere rock ocean weathering rivers We’ve already examined why water is a powerful Solvent, now let’s look at the whole picture Ocean Chemistry and the Geochemical Cycle The Ocean has Both Inputs and Outputs Outputs include: 1--sea salt aerosols 2--biogenic sediments (biological processes); deposited on ocean floor (CaCO3, SiO2) 3--inorganic sediments (precipitates, evaporites; adsorption) 4--interaction of seawater with hot basalt (Mg and SO4 "sink”) Outputs compete with Inputs to shape the chemistry of seawater Outputs: Seawater Evaporation in isolated basins. These sediments (“evaporites”) provide a record of seawater chemistry Salt from the Sea Dead Sea Salt, Evaporation! At work The Grand Geochemical Cycle: Residence time Let’s consider: The average time that a substance remains dissolved in seawater We call this the “residence time” of an element or substance Residence Time (yrs.) = Total amount in seawater (kg) Input rate (kg/yr) where Input rate= average concentration in rivers (kg/km3) x river discharge (km3/yr) We will see how this works: first for water, then for total salt, and, finally, for some individual elements. These calculations give us insights into how the system works Residence time of water in the ocean Volume = 1.4 x 109 km3 How long does it take to cycle ocean water through rivers and back again? River Influx = 3.7 x 105 km3 /yr t = Volume / Influx 1.4 x 109 km3 t = 3.7 x 105 km3 t = 4000 years The Grand Geochemical Cycle •How much time to make the ocean salty? •about 5 x 1022 grams of dissolved solids in ocean •rivers bring in about 2.5 x 1015 gm dissolved solids per year --think about it! •Should only take about 2 x 107 years (20 million yrs.) to bring oceans to present salinity Assuming: •rivers have kept approx. same input through time •oceans have kept approx. same composition through time --but we know oceans are 3.8 billion yrs. old •This confirms that there must be output of material from ocean!! The Grand Geochemical Cycle Typical Element Residence Times Cl 80 million yrs. SO4 9 million yrs. Na 60 million yrs. Ca 1 million yrs. Mg 10 million yrs. PO4 100 thousand yrs. Don’t worry too much about absolute numbers, but be able to explain why Cl residence time is so much longer than, say, that of phosphate The Grand Geochemical Cycle Residence time is inversely related to extent of involvement in chemical reactions in the ocean •Na and Cl primarily precipitate as evaporite deposits (infrequent events over geologic history). Bio-inert •Ca used by organisms to make CaCO3 (calcium carbonate) skeletons •PO4 used in biological cycle (organic matter production)--this is a nutrient element. Biolimiting Was the Chemistry of the Ancient Oceans the Same as Today? 35 0/00 Ocean Salinity We can use ancient evaporite deposits to tell us how ocean chemistry changed through time (different sequence of minerals precipitated) We also can use the chemistry of “brine” inclusions in the evaporites to constrain elemental ratios of major elements in seawater through time. New data suggest that ocean chemistry has changed a bit through time. Perhaps this reflects changes in ocean basin spreading rates and cycling of seawater through hydrothermal systems! Time (billions of yrs)