Chem Midterm Review
advertisement

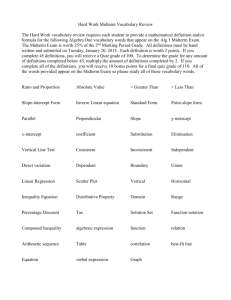
Mrs. Gebhard Chemistry (422) Midterm Review STUDY!!!! **Refer to your note packets/books for help with this material CH1 Chemical Exploration: 1. a. Develop a Hypothesis for the experiment above. b. Determine the Independent (Manipulated) Variable: c. Determine the Dependent Variable: d. Determine the Experimental Control: 2. What is Chemistry? CH 2: Matter and Energy: 1. Name something that is not matter: 2. Define: Extensive Property: Intensive Property: 3. Pure Substance vs. Mixtures: What is the main difference? Name________________________ Mrs. Gebhard Chemistry (422) Midterm Review Name________________________ 4. Classify each of the following as an element (E), compound (C), solution (S), or heterogeneous mixture (HET) Copper _____________________ Brass _____________________ Maple Syrup _____________________ Granite Rock _____________________ Italian Dressing _____________________ Carbon Dioxide _____________________ 5. States of Matter Solid __________________ Shape __________________ Volume Liquid __________________ Shape __________________ Volume Gas __________________ Shape __________________ Volume 6. Chemical vs. Physical Properties and Changes: a. Describe the difference between physical properties and chemical properties. Give an example of each. b. Describe the difference between physical change and chemical change. Give an example of each. 7. Identify each of the following changes as chemical (CC) or physical (PC). _________________: grinding a crystal into powder _________________: rusting of iron _________________: digesting an apple _________________: mixing oil and water _________________: bleaching a piece of cloth _________________: melting iron _________________: tarnishing of a silver necklace 8. Identify whether the property is chemical(CP) or physical (PP) _________________: the odor of hydrogen cyanide is sweet _________________: sodium will react with water in air _________________: the volume of a typical milk jug is one gallon _________________: gold is an excellent conductor of electricity _________________: propane is extremely flammable _________________: talc is finely powdered _________________: the density of lead is 13.5 g/mL _________________: oxygen is needed for objects to burn _________________: uranium is radioactive CH3: Measurement and Problem Solving: 1. Significant Figures 6.751 g 30.07 g 0.156 kg 0.106 cm 2500 mL 0.0230 L 28.0 m 0.0067 g 54.052 cm3 0.1209 m 43.09 cm 50,000 mL Mrs. Gebhard Chemistry (422) Midterm Review Name________________________ 2. Addition/Subtraction Rules: 8.76m + 6.0m = 2222km + 111km = 3. Multiplying/Dividing Rules: 2.2mm * 5.00mm = 6.42cm/2.1cm = 4. Scientific Notation A. 0.00003 B. 56,000,000 C. 650 D. 0.00007 E. 43,500 F. 0.00568 G. 5.63x103 H. 3.71x104 I. 8.5x106 J. 6.4x10-2 K. 5.63x10-5 L. 9.3x10-1 5. Accuracy and Precision a. Definition of accuracy: b. Definition of precision: 6. Units: Convert between Celsius and Kelvin temperatures 34.1˚C 45˚C 120˚C 653K 320K 283K 7. Mass vs. Weight: What is the difference? Mrs. Gebhard Chemistry (422) Midterm Review Name________________________ 8. Factor Label Problems 1 yard = 3 feet 1 gallon = 3.97 liters 1 foot = 12 inches 1 mile = 5280 feet 1 inch = 2.54 cm 100 cm = 1 meter 1 mile = 1.6 km 1 liter = 1000 cm3 Given metric prefixes (not equalities) “milli (m) 1000 times smaller than the unit it precedes” → 1000mm = 1m Use the above conversion factors to solve the following problems. A) Calculate the number of centimeters in 4.5 yards? B) Convert 2.1 kilometers into inches. C) The density of lead (Pb) is 11.34 g/cm3. Find the density in kg/L. 9. Density A) Define: Mass:______________________________________________________ Measuring Instrument?___________________ Units? _____________________ B) Define: Volume:____________________________________________________ Measuring Instrument? ___________________ Units? _____________________ OBJECT MEASUREMENT METHOD MEASURING INSTRUMENT Liquid Block of Wood Irregular Shaped Object 10. Density Equation: 11. Calculate the volume of 80.0g of ether if the density of ether is 0.70 g/mL. Will ether sink or float in water (1.00g/mL)? Mrs. Gebhard Chemistry (422) Midterm Review Name________________________ CH 4: Atomic Theory **Use your Atomic Theory Study Guide for information about the scientists, experiments and atom models related to atomic theory. Chart Terms (like the tables, but they will be multiple choice) PARTICLE CHARGE LOCATION MASS Proton Electron Neutron Substance Symbol Atomic Number Mass Number Number of Protons Magnesium Krypton Calcium Silver MIDTERM INQUIRY: Percent Sugar Lab % Sugar Standard Calculations Density of Trials Average Density Density vs. % Sugar Graph (Calibration Curve) Use the calibration curve to find the % Sugar of unknown solutions Write a hypothesis Independent variable Dependent Variable What was the control? Why multiple trials? Purpose of calibration curve Number of Electrons Number of Neutrons