Chemistry Midterm Study Guide
advertisement

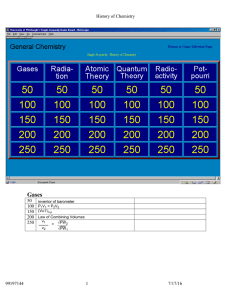
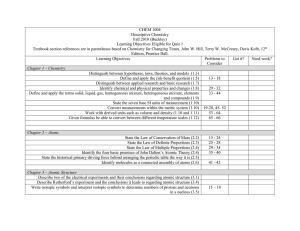
Midterm Exam Study Guide Chemistry, 2015 The midterm exam will include content from Chapters 1,2,3,4,5,6 of your textbook. When studying, you should review how the terms below relate to your tests and quizzes from each of these chapters. • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • Organic vs inorganic chemistry Biochemistry Physical vs analytical chemistry Mixture vs. compound Vapor vs. gas Distillation vs. filtration Mass Homogenous vs heterogenous Matter Reactant vs. product Chemical reaction Phase Element Technology Physical vs chemical change Lavoisier vs. Flemming Hypothesis vs theory Importance of collaboration and communication in chemistry Independent vs. dependent variables Period vs. groups in the periodic table Solids vs. liquids vs. gases Law of Conservation of Mass Extensive vs. intensive properties Definition of a chemical property Structure of an atomic nucleus Contributions of the following people: Rutherford, Thomson, Dalton, Democritus, and Goldstein Determining the number of neutrons Isotopes Atomic mass vs. atomic number How to determine atomic number Speed of light # of electrons in each electron orbital (s, p, d) Amplitude vs. frequency Spectrum of visible light Bohr’s model Pauli exclusion principle Aufbau’s digram Protons, neutron, and electrons Metals vs. nonmetals vs. metalloids • • • • • • • • • • Which groups alkali metals and alkaline earth metals belong to Periodic Law Representative elements Noble gases Atoms Cations vs Anions Ionization energy trends Electronegativity trends Nuclear energy trends Mendeleev