first week assignments_directions
advertisement

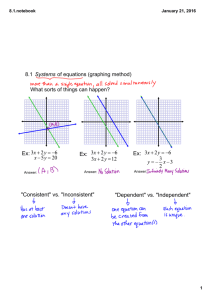
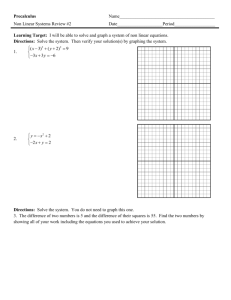
AP CHEMISTRY 1st Week ASSIGNMENTS/TASKS CHECKLIST 1. Getting to Know You—google forms. Due Thursday, 9/10/15 Go to Mrs. Varma’s school website (marsd.org HS Staff Varma). On the welcome page, scroll to the bottom and click on the link for google forms. You must login with your marsd.org account in order to access the “Getting to Know You” form. Complete by due date given above. 2. Classroom Rules and Expectations. Bottom of the last page must be filled in and returned by Thursday, 9/10/15. Read the Classroom Rules and Expectations document carefully. It contains very important information about the course, grading policies, expectations etc. We will discuss more in the next few days. 3. Safety Contracts. Signed by you and your parents in INK. Due Wednesday, 9/16/15 You have two copies of the safety contract. BOTH must be signed. One must be turned in BEFORE you are allowed to carry out any lab experiments. The other is to be kept for reference in your Chemistry binder. We will discuss in great detail. 4. Safety Quiz Expect to take the safety quiz between 9/16 to 9/18. You will take the safety quiz after we have discussed the safety contract in detail, along with safety equipment in the classroom, and other safety considerations. 5. Materials needed for class: 3 ring binder loose leaf paper writing utensils calculator (scientific, does not need to be graphing) book cover sheet protectors (~10) 6 tabbed dividers Thread bound Graphing Notebook (see sample) 6. Setting up 3-ring binder It is imperative that you be organized in this course. You will be getting a lot of handouts, reference materials, worksheets and labs. If you are not organized, you will quickly find yourself overwhelmed by trying to find the right materials at the right time. For this reason, I require that you set up your 3-ring binder in the following order using tabbed dividers: a. Notes/Do Nows/Exit Tickets b. Homework c. Tests/quizzes d. Labs/Activities e. Vocabulary f. Reference Materials (start with the following) i. Periodic Table (bring your own, or ask NOW ) ii. Solubility Rules (provided online, MEMORIZE) iii. Polyatomics /Common ions (provided online, MEMORIZE) iv. Transition Elements oxidation states (provided online, MEMORIZE) v. AP Chem Reference Sheets (provided) vi. Start your own EQUATIONS Reference section. Keep a list of ALL mathematical equations we use, including symbols, conversions, units etc. 1. Frequently tally against reference sheet provided by College Board to ensure you know which ones appear on the Equations and Constants sheet and which other ones you may need to memorize. vii. CHEMICAL EQUATIONS reference table. Start a second reference section with sample chemical equations (types/groups) that commonly appear on AP test. ALL work must be dated and kept in chronological order. There may be periodic notebook checks. There may also be periodic open notes quiz based on a prior HW, do now or exit ticket. It will help you considerably if you are able to find the relevant material(s) quickly. Work handed in without proper headers (name, date, period, class, title) will have points deducted. 7. Textbooks You may keep your textbook at home. On occasion when it is required, I will give you prior notice to bring the textbook to class. It must be covered in order to avoid undue damage. 8. Labs Lab Notebook (graphing/thread bound)—see attached LAB NOTEBOOK SETUP INSTRUCTIONS (also available on Mrs. Varma’s website) Labs will start on Wednesday, 9/16/15. You must have a thread-bound graphing notebook prior to your first lab period. We will discuss specific lab notebook setup on your first lab day. The first three labs are as follows a. Lab Equipment b. Proper Procedures c. Percent Composition of Silver Oxide 9. Quizzes and Tests a. Quiz 1: Wednesday 9th, 2015--**This is the second day of school i. Nomenclature, molar mass, percent composition, empirical/molecular formulas, significant figures, scientific notation, metric units/conversions. NO REFERENCE SHEETS except periodic table. **YOU MUST PASS THIS QUIZ WITH ATLEAST AN 85%. b. Chapter 1-3 Test: Friday Sept 11th, 2015--**First Friday ?? i. Topics: (we will discuss more in class) 1. Periodic Table basics a. Properties based on location, states of matter, general reactivity etc. 2. Stoichiometry a. Mol-mass, mol-volume, mol-particle, limiting reagent, percent yield 3. Chemical equations a. Predicting products, balancing, states of matter 10. Weekly Quiz MONDAYS (unless test on Monday)— 4-7 M.C Questions (3-4 on previous week’s topic, 2-4 on ANY topic from beginning of year) OR 1 open ended. TIMED_7-10 minutes tops. a. Based on actual AP questions--helps familiarize you with questioning style. 11. Review Guide: Plan on buying a review guide (Princeton, Barron’s, Kaplan. REA Crash Course etc.) and USE it as we progress through topics. You should probably wait for a while though, since we don’t know if a newer edition will be available.