Pressure - Physics
advertisement

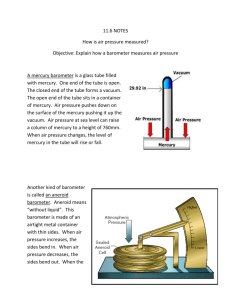
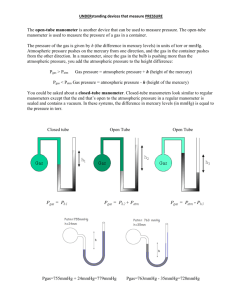
Pressure 1.7. David Raju Vundi Pressure Pressure is the amount of force applied to a known area. We can calculate it using the equation Pressure = Force (N) / Area (m2) We can reduce pressure by reducing the size of the force or increasing the size of the area. Pressure and Density Density = Mass/Volume A property of the material. Pressure = Force/Area Depends on the height of the fluid. Same in all directions. Units are: Force/Area = N/m2. Pascals 1 Pa = 1 N/m2. Atmosphere 1 atm = 1.013 X 105 N/m2. 3 Pressure is due to the net force of the molecules in a fluid colliding with the walls. A very large number of collisions happen each second. Each collision exerts a tiny net force on the wall. How to Measure Pressure Barometer – measures atmospheric pressure vacuum Patm PHg Aneroid Barometer Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem vacuum mercury (Hg) air pressure barometer: device to measure air pressure Barometer Empty space (a vacuum) Hg Weight of the mercury in the column Weight of the atmosphere (atmospheric pressure) Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 401 Water column (34.0 ft. high or 10.4 m) Barometer Mercury filled 760 mm = 1 atm Atmospheric pressure Water filled 10400 mm = 1 atm Mercury column (30.0 in. high or 76 cm) The barometer measures air pressure Barometers Mount Everest Sea level Sea level On top of Mount Everest Pressure Experiment 100g I think the bigger mass will sink further I think a bigger area will stop the mass sinking SAND Torricelli Barometer The mercury in the tube pushes Weight of mercury down with its weight. The bottom of the tube is open to the atmosphere. The air pushes on the open surface of the mercury. On an average day, the pressure of the air equals the pressure exerted by a column of mercury 760 mm high. Above 760 mm, there is a vacuum in the tube. Barometer Detail Why doesn’t the diameter of the column of Hg make a difference? Recall that Pressure = force/area. The “force” is the weight of the mercury, but the pressure that results is that weight divided by the area of the column. So … a bigger column weighs more but also has a proportionally bigger area, and the two factors cancel one another out. The pressure caused by the column of mercury pressing down is independent of the diameter of the column. Manometer PHg A manometer is comprised of a bulb containing a gas and a U-shaped tube. The U-shaped tube is partially filled with mercury. The weight of the mercury puts pressure on the gas. If the U-tube is OPEN there is also air pressure acting on the gas. The gas molecules put pressure on the mercury. Closed Manometers There is a balance vacuum PHg between the weight of the mercury on the left (PHg) and the pressure of the gas on the right (Pgas). The difference between the heights of the mercury on each side of the tube is a measure of the pressure of the gas. Pgas = Dh Open Manometers When gas pressure is greater Pair PHg than atmospheric pressure, the mercury is pushed toward the open end. The balance is between the gas on the right, and the air plus mercury on the left. Pair + PHg = Pgas The weight of the mercury is measured as the height difference: PHg = Dh So Pgas = Pair + Dh Open Manometers When gas pressure is less than Pair PHg atmospheric pressure, the mercury is pushed toward the gas reservoir. The balance is between the air on the left and the gas plus mercury on the right: Pair = Pgas + PHg The weight of the mercury is measured as the height difference: PHg = Dh So Pair = Pgas + Dh Or Pgas = Pair- Dh Sample Problems 1. Pair = 790 mm Dh = 20 mm 2. Dh = 13 mm PAIR = 753 mm 3. PAIR = 765 mm Dh = 27 mm Find the pressure of the gas in each manometer. Pay attention to whether the manometer is open or closed! Sample Problem Answers 1. Pgas+ Dh = Pair Pgas= 790 mm - 20 mm = 770 mm Hg 2. Pgas = vacuum + 13 mm = 13 mm Hg 3. Pgas = Pair + Dh Pgas = 765 + 27 = 792 mm Hg