emi412227-sup-0002-fs2
advertisement
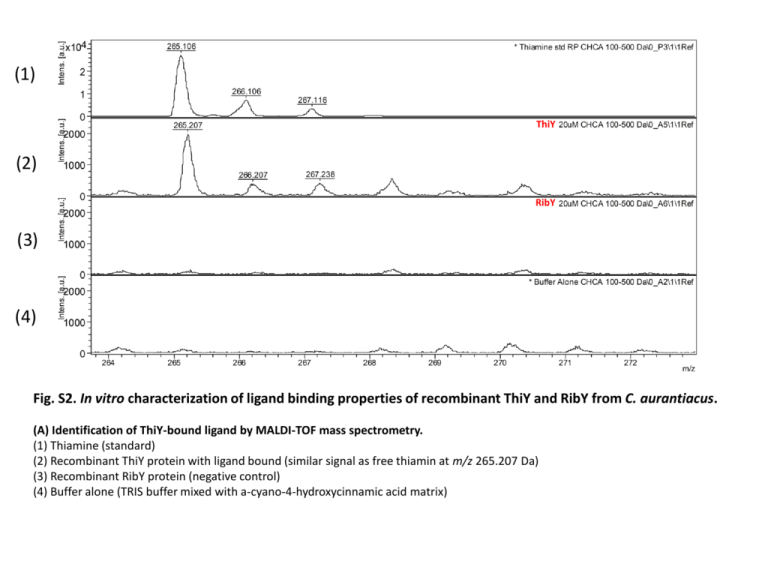
(1) ThiY (2) RibY (3) (4) Fig. S2. In vitro characterization of ligand binding properties of recombinant ThiY and RibY from C. aurantiacus. (A) Identification of ThiY-bound ligand by MALDI-TOF mass spectrometry. (1) Thiamine (standard) (2) Recombinant ThiY protein with ligand bound (similar signal as free thiamin at m/z 265.207 Da) (3) Recombinant RibY protein (negative control) (4) Buffer alone (TRIS buffer mixed with a-cyano-4-hydroxycinnamic acid matrix) Fig. S2. In vitro characterization of ligand binding properties of recombinant ThiY and RibY from C. aurantiacus. (B) Binding of vitamin ligands tested by fluorescence-based thermal shift (FTS) assay. The positive difference in melting temperatures (ΔTm, °C) between a protein incubated without a ligand and a protein incubated with an increasing ligand concentration was interpreted as potential ligand binding. Points on the plot represent means, and error bars indicate standard deviation values of three independent measurements. 0.6 0.5 Absorbance 0.4 Ligand 0.3 RibY + ligand Riboflavin 0.2 0.1 0 300 350 400 450 500 Wavelength (nm) Fig. S2. In vitro characterization of ligand binding properties of recombinant ThiY and RibY from C. aurantiacus. (C) UV-visible spectra of the recombinant RibY protein and its co-purified ligand. Dark blue, RibY protein with bound co-purified ligand, which is similar to the previously described spectrum of 6,7-dimethyl-8ribityllumazine (Kaiser et al., 2007). Green, after extraction from the protein by urea denaturation, the RibY ligand showed a similar spectrum. Light blue, free riboflavin (standard). Fig. S2. In vitro characterization of ligand binding properties of recombinant ThiY and RibY from C. aurantiacus. (D) Identification of RibY-bound ligand by MALDI-TOF mass spectrometry. (1) (2) RibY (3) (1) Buffer alone (TRIS buffer mixed with a-cyano-4-hydroxycinnamic acid) (2) Riboflavin (standard). Three peaks at 377, 378 and 379 Da correspond to the following forms of riboflavin: oxidized and protonated ([376 + 1H] +), protonated riboflavin radical ([376 + 2H]+) and protonated reduced riboflavin ([376+3H]+) (Ohashi, Itoh 2003). (3) Recombinant RibY protein with bound 6,7-dimethyl-8-ribityllumazine. No peaks at the same mass as for riboflavin were detected. Fig. S2. In vitro characterization of ligand binding properties of recombinant ThiY and RibY from C. aurantiacus. (D) Identification of RibY-bound ligand by MALDI-TOF mass spectrometry. (1) RibY (2) (3) (1) Riboflavin (standard). No peaks at 326, 327, 328, 329 Da. (2) Recombinant RibY protein with bound 6,7-dimethyl-8-ribityllumazine. Three peaks at 327, 328, and 329 Da correspond to the following forms of 6,7-dimethyl-8-ribityllumazine: oxidized and protonated ([326 + 1H]+), protonated radical ([326 + 2H]+), and protonated reduced ([326+3H]+). The profile has main peak at 328 Da, with the very close to riboflavin distribution of three forms. By analogy with riboflavin, the protonated radical ([326 + 2H]+) was the most abundant form. (3) Buffer alone (TRIS buffer mixed with a-cyano-4-hydroxycinnamic acid).



