Crystal Structure
advertisement

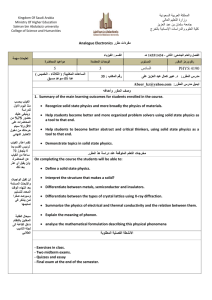
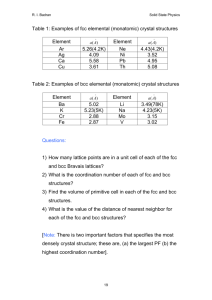
Why Study Solid State Physics? Ideal Crystal • An ideal crystal is a periodic array of structural units, such as atoms or molecules. • It can be constructed by the infinite repetition of these identical structural units in space. • Structure can be described in terms of a lattice, with a group of atoms attached to each lattice point. The group of atoms is the basis. Bravais Lattice • An infinite array of discrete points with an arrangement and orientation that appears exactly the same, from any of the points the array is viewed from. • A three dimensional Bravais lattice consists of all points with position vectors R that can be written as a linear combination of primitive vectors. The expansion coefficients must be integers. Crystal lattice: Proteins Crystal Structure Honeycomb: NOT Bravais Honeycomb net: Bravais lattice with two point basis Crystal structure: basis Translation Vector T Translation(a1,a2), Nontranslation Vectors(a1’’’,a2’’’) Primitive Unit Cell • A primitive cell or primitive unit cell is a volume of space that when translated through all the vectors in a Bravais lattice just fills all of space without either overlapping itself or leaving voids. • A primitive cell must contain precisely one lattice point. Fundamental Types of Lattices • Crystal lattices can be mapped into themselves by the lattice translations T and by various other symmetry operations. • A typical symmetry operation is that of rotation about an axis that passes through a lattice point. Allowed rotations of : 2 π, 2π/2, 2π/3,2π/4, 2π/6 • (Note: lattices do not have rotation axes for 1/5, 1/7 …) times 2π Five fold axis of symmetry cannot exist Two Dimensional Lattices • There is an unlimited number of possible lattices, since there is no restriction on the lengths of the lattice translation vectors or on the angle between them. An oblique lattice has arbitrary a1 and a2 and is invariant only under rotation of π and 2 π about any lattice point. Oblique lattice: invariant only under rotation of pi and 2 pi Two Dimensional Lattices Three Dimensional Lattice Types Wigner-Seitz Primitive Cell: Full symmetry of Bravais Lattice Conventional Cells Cubic space lattices Cubic lattices BCC Structure BCC Crystal BCC Lattice Primitive vectors BCC Elements with BCC Structure Summary: Bravais Lattices (Nets) in Two Dimensions Escher loved two dimensional structures too Summary: Fourteen Bravais Lattices in Three Dimensions Fourteen Bravais Lattices … FCC Structure FCC lattice Primitive Cell: FCC Lattice FCC: Conventional Cell With Basis • We can also view the FCC lattice in terms of a conventional unit cell with a four point basis. • Similarly, we can view the BCC lattice in terms of a conventional unit cell with a two point basis. Elements That Have FCC Structure Simple Hexagonal Bravais Lattice Primitive Cell: Hexagonal System HCP Crystal Hexagonal Close Packing HexagonalClosePacked HCP lattice is not a Bravais lattice, because orientation of the environment Of a point varies from layer to layer along the c-axis. HCP: Simple Hexagonal Bravais With Basis of Two Atoms Per Point Miller indices of lattice plane • The indices of a crystal plane (h,k,l) are defined to be a set of integers with no common factors, inversely proportional to the intercepts of the crystal plane along the crystal axes: Indices of Crystal Plane Indices of Planes: Cubic Crystal 001 Plane 110 Planes 111 Planes Simple Crystal Structures • There are several crystal structures of common interest: sodium chloride, cesium chloride, hexagonal close-packed, diamond and cubic zinc sulfide. • Each of these structures have many different realizations. NaCl Structure NaCl Basis NaCl Type Elements CsCl Structure CsCl Basis CsCl Basis CeCl Crystals Diamond Crystal Structure ZincBlende structure Symmetry planes The End: Chapter 1 Bravais Lattice: Two Definitions The expansion coefficients n1, n2, n3 must be integers. The vectors a1,a2,a3 are primitive vectors and span the lattice. HCP Close Packing HCP Close Packing Close Packing 2 Close Packing 3 Close Packing 4 Close Packing 5 NaCl Basis Close Packing of Spheres