pyogenic cocci
advertisement

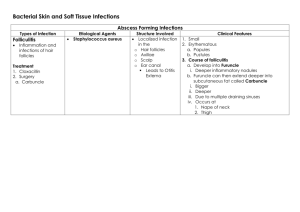
There are two medically important genera of gram positive cocci: Staphylococcus and Streptococcus. Staphylococcal infections range from the trivial to the rapidly fatal. They can very difficult to treat, especially those contracted in hospitals, because of the remarkable ability of Staphylococci to become resistant to antibiotics. Staphylococci are ubiquitous in nature, with about a dozen species occurring as a part of human flora. The most virulent of the genus, S.aureus , is one of the most common causes of bacterial infections Staphylococci & Streptococci are non motile & do not form spores. Both Staphylococci & Streptococci are gram positive cocci, but they are distinguished by two main criteria. *Microscoplly, Staphylococci appear in grape-like clusters, whereas Streptococci are in chains. *Biochemically, Streptococci produce catalase (ie, they degrade hydrogen peroxide), whereas Streptococci do not. General features of Staphylococci Staphylococci generally stain darkely gram positive, they are round &tend to occur in bunches like grapes. They are true facultatively anaerobic org. They produce catalase. The most virulent spp. of Staphylococcus is S.aureus, almost all isolates of which secrete coagulase-an enzyme that causes citrated plasma to clot. There are other spp. that occasionally cause disease; these lack coagulase & are often referred to as coagulase negative Staphylococci Staphylococcus aureus S.aureus disease may be: *Largely or wholly the result of actual invasive infection (that is, colonization), overcoming host defense mechanisms, & the production of extracellular substances which facilitate invasion. *A result of toxins in the absence of invasive infection. or *A combination of invasive infection and intoxication. Epidemiology S.aureus is frequently carried by healthy individuals on the skin & mucous membranes. Carriers serve as a source of infection themselves & others; ex, by direct contact, by contamination of fomites or food, which can then result in food poisoning. Pathogensis S.aureus causes disease by infecting tissues typically creating abscesses and/or by producing toxins. A common entry point into the body is a break in the skin. Another portal of entry is the respiratory tract. The localized host response to Staphylococcal infection is inflammation, characterized by swelling, accumulation of pus, and necrosis of tissue. Fibroblasts and their products may form a wall around the inflamed area, which contains bacteria & leukocytes. This creates a characteristic, pus-filled boil or abscess. Serious consequences of staphylococcal infections occur when the bacteria invade the bloodstream. The resulting septicemia may be rapidly fatal. Bacteremia may result in seeding internal abscesses, skin lesions, or infections in the lung , kidney, heart, skeletal muscle, or meninges. Pathogenic virulence factors are the genetic, biochemical, or structural features that enable an organism to produce disease. The clinical outcome of an infection depends on the virulence of the pathogen and the opposing effectiveness of the host defense mechanisms. S.aureus produce many enzymes include coagulase, fibrinolysin, hyaluronidase, proteases, nucleases, & lipases. Also these orgs expresses many potential virulence factors as follows: Cell wall virulence factors a) Protein A is a major component of the S.aureus cell wall. It binds to the Fc moiety of IgG, exerting an antiopsonin( and therefore strongly antiphagocytic) effect. b) Fibronectin-binding protein (FnBP) and other staphylococcal surface proteins promote binding to mucosal cells and tissue matrices. 2-Cytolytic exotoxins: α, β, γ, and δ toxins attack mammalian cell (including red blood cell) membranes, and are often referred to as hemolysins. 3-Superantigen exotoxins: These toxins have affinity for the T cell receptor-MHC Class II antigen complex. They stimulate enhanced T lymphocyte response. This major T cell activation can cause toxic shock syndrome, primarily by release into the circulation of inordinately large amounts of T cell cytokines, such as interleukin-2 (IL-2), interferon- γ (IFN- γ), and tumor necrosis factor- α (TNF- α). Enterotoxins: Enterotoxins (six major antigenic types: A, B, C, D, E, and G) are produced by approximately half of all S.aureus isolates. When these bacteria contaminate food and are allowed to grow, they secrete enterotoxin, ingestion of which can cause food poisoning. Enterotoxins are superantigens that are even more heat stable than S.aureus; therefore, organisms are not always recovered from incriminated food Toxic shock syndrome toxin (TSST-1) is the classic cause of toxic shock syndrome. Because of similarities in molecular structure, it is sometimes referred to as staphylococcal enterotoxin F (SEF), although it does not cause food poisoning when ingested. Exfoliatin (exfoliative toxin, ET) is also a superantigen. It causes scalded skin syndrome in children Clinical findings The important clinical manifestations caused by S.aureus can be divided into two groups: inflammatory & toxin-mediated. 1-Inflammatory Skin infections, such as impetigo, furuncles, carbuncles, folliculitis…..etc. Septicemia can originate from any localized lesion, especially wound infection, or as a result of intravenous drug abuse. Endocarditis on normal or prosthetic heart valves. Osteomyelitis and arthritis Pneumonia in postoperative patients or following viral respiratory infection. Abscesses can occur in any organ when the organism circulates in the bloodstream (bacteremia). Toxin-mediated Food poisoning caused by ingestion of enterotoxin, which is performed in foods & hence has a short incubation period (1-8 hours). Toxic shock syndrome is characterized by high fever, hypotension, rash, vomiting, diarrhea & multiorgan involvement (especially GI, renal, &/or hepatic damage). TSST causes toxic shock , especially in tampon-using menstruating women or in individualswith wound infections. Toxic shock also occur in patients with nasal packing used to stop bleeding from the nose. TSST is produced locally by S.aureus in either the vagina, nose, or other infected site. The toxin enters the bloodstream, causing a toxemia. Scalded skin syndrome is characterized by fever, large bullae resulting from the action of an exfoliative toxin that attacks the intracellular adhesive of the stratum granulosum causing marked epithelial desequamation.This syndrome occurs most often in young children Lab. Diagnosis Specimen: There are to be collected depending on the nature of lesion, pus from suppurative lesions, CSF from meningitis, blood from septicemia, sputum from respiratory infection and suspected food, vomit or faeces from food poisoning. Smear: Examination of gram stained smear of pus & other specimens shows gram positive cocci in clusters with some single & paired cocci. Culture : Specimens inoculated on blood agar or mannitol agar (selective media for Staph.aureus show typical colonies on 18 hr incubation at 37 .ْْC Coagulase test: the coagulase positive strain is identified . Typing test. Coagulase negative staphylococci There are two coagulase negative staphylococci of medical importance: S.epidermidis & S. saprophyticus. S.epidermidis infections are almost always hospital acquired, whereas S. saprophyticus infections are almost always community- acquired. S.epidermidis is part of the normal human flora on the skin & mucous membranes but can cause infections of intravenous catheters & prosthetic implants, eg, heart valves (endocarditis), vascular grafts, & joints. S.epidermidis is also a major cause of sepsis in neonates & of peritonitis in patients with renal failure who are undergoing peritoneal dialysis through an indwelling catheter. It is the most common bacterium to cause cerebrospinal fluid shunt infections S.epidermidis produces an extracellular polysaccharide material (sometimes called"slime") that facilitates adherence to bioprosthetic material surfaces, such as intravenous catheters, & acts as a barrier to antimicrobial agents. S. saprophyticus causes urinary tract infections, particularly in sexually active young women. Streptococci Streptococci are G+ve, non motile, & catalase-negative. Clinically important genera include Streptococcus & Enterococcus. They are ovoid to spherical in shape, & occur as pairs or chains. Because of their complex nutritional requirements, blood-enriched medium is generally used for their isolation. Classification 1-Hemolytic classification α- hemolytic streptococci cause a chemical change in the hemoglobin of red cells in blood agar, resulting in the appearance of green pigment that forms a ring around the colony. β - hemolytic streptococci cause gross lysis of red blood cells, resulting in a clear ring around the colony. Two types of β – hemolysins are released. Streptolysin O (inactivated by atmospheric oxygen) is demonstrable only in deep colonies while Streptolysin S ( oxygen stable) is responsible for surface colony haemolysis. γ- hemolytic is a term applied to streptococci that cause no color change or lysis of red blood cells. There are two important antigens of beta- hemolytic streptococci: C- carbohydrate determines the group of β hemolytic streptococci. It is located in the cell wall, & its specificity is determined by an amino sugar M protein is the most important virulence factor & determines the type of group A β hemolytic streptococci. It protrudes from the outer surface of the cell & interferes with ingestion by phagocytes (antiphagocytic). There are approximately 80 Grifith serotypes based on the M protein, which explains why multiple infections with S.pyogenes that produce certain M protein types are rheumatogenic ( cause primarily rheumatic fever ), whereas strains of S.pyogenes that produce other M protein types are nephritogenic (cause primarily acute glomerulonephritis). Although M protein is the main antiphagocytic component of S.pyogenes, the org also has a polysaccharide capsule that play a role in retarding phagocytosis. There are approximately 80 Grifith serotypes based on the M protein, which explains why multiple infections with S.pyogenes that produce certain M protein types are rheumatogenic ( cause primarily rheumatic fever ), whereas strains of S.pyogenes that produce other M protein types are nephritogenic (cause primarily acute glomerulonephritis). Although M protein is the main antiphagocytic component of S.pyogenes, the org also has a polysaccharide capsule that play a role in retarding phagocytosis. Serological classification (Lancefield) Many species of streptococci have a polysaccharide in their cell walls known as Ccarbohydrate. The Lancefield scheme classifies primarily β hemolytic streptococci into groups A-U on the basis of their C- carbohydrate. The clinically most important groups of β hemolytic streptococci are types A & B. Classification based on Schleifer & Kilpper-Balz This classification depend on the basis of structure of the cell wall peptidoglycan together with the G+C content of the DNA & the results of DNA pairing. They have divided the genus Streptococcus into sex groups Pyogenic streptococci – S.pyogenes Pneumococci – S.pneumoniae Oral streptococci – S.mutans Enterococci – S. faecalis Lactic streptococci – S.lactice Other streptococci – S.bovis Group A β-hemolytic streptococci S.pyogenes, the most clinically important member of this group of gram positive cocci, is one of the most frequently encountered bacterial pathogens of humans worldwide. It can invade apparently intact skin or mucous membranes, causing some of the most rapidly progressive infections known. A low inoculum suffices for infection. The growth of S.pyogenes is inhibited by antibiotic bacitracin, an important diagnostic criterion. Structure 1-Capsule (antiphagocytic) 2-Cell wall a)Fimbriae: the fimbriae contain the major S.pyogenes virulence factor, M protein. b)Group A-specific C-carbohydrate c)Protein F ( fibronectin- binding protein) 3-Extracellular products Epidemiology The only reservoir for S.pyogenes in nature is the skin & mucous membranes of the human host. Respiratory droplets or skin contact spread Group A streptococcal infection from person to person, especially in crowded environments such as classrooms or children's play area. Pathology S.pyogenes cells, perhaps in an inhaled droplet, attach to the pharyngeal mucosa via actions of protein F, lipoteichoic acid, & M protein. The bacteria may simply colonize; the patient is then considered colonized. Alternatively, bacteria may grow & secrete toxins, causing damage to surrounding cells, invading the mucosa, and eliciting an inflammatory response with attendant influx of white cells, fluid leakage, & pus formation. The patient then has streptococcal pharyngitis. Occasionally, there is sufficient spread that the blood stream is significantly invaded, possibly resulting in septicemia &/or seeding of distant sites, where cellulitis (acute inflammation of subcutaneous tissue), fasciitis (inflammation of the tissue under the skin that covers a surface of underlying tissue), or myonecrosis (death of muscle cells) may develop rapidly or insidiously. Clinical findings 1-Pyogenic diseases a-Sore throat ( acute tonsillitis/ & pharyngitis) is the commonest of streptococcal diseases. The org may spread to surrounding tissue causing complication like otitis media, mastoiditis,sinusitis, meningitis, peritonitis & pneumonia b-Skin infection Local infections in superficial layers of skin due to S.pyogenes include impetigo & erysipelas. c-Other pyogenic diseases * puerperial sepsis ( postpartum infection of uterus). *Sepsis: Infection of wounds, burns, chronic skin lesion (eczema, psoriasis). *Lymphadenitis, septicaemia, acute endocarditis, abscess in internal organs (brain, liver, lung, & kidney). 2- Toxigenic diseases a-Scarlet fever Scarlet fever occurs as a complication of streptococcal infection (sore throat) when the infecting strain produces erythrogenic toxin & the patient has got no antitoxic immunity. b- Streptococcal toxic shock syndrome This syndrome is mediated by pyrogenic exotoxins that function as superantigens causing massive, nonspecific T- cell activation & cytokines release. It is similar to staphylococcal toxic shock syndrome. 3-Immunogenic diseases ( non suppurative infection) a- Acute glomerulonephritis(AGN): This rare, postinfectious sequela occur as soon as one week after impetigo or pharyngitis ensues, due to a few nephritogenic strains of group A streptococci. Antigen-antibody complexes on the basement membrane of the glomerulus initiate the disease. The most clinical features are hypertension, edema of the face and ankles, & smoky urine (due to red cells in the urine). b-Acute rheumatic fever: this autoimmune disease occurs two to three weeks after the initiation of pharyngitis. It is caused by cross reactions between antigens of the heart & joint tissues, & the streptococcal antigen (especially the M protein epitopes). It is characterized by fever, rash, carditis, & arthritis. Lab. Diagnosis 1-Specimen: Throat swab, pus& lesion samples, sputum, blood , spinal fluid 2-Smear: G+ve cocci in chains or pairs are found association with pus cells. 3-culture: Specimen should be inoculated immediately. Specimen is inoculated in the blood agar for overnight. ْmedium & incubated at 37C Hemolysis develops better under anaerobic conditions or under 5-10% CO2. The bacterial colonies are small, dry & surrounded by β-hemolysis. A simple technique of detection of S.pyogenes ( group A) is done by agar plate test using paper discs impregnated with bacitracin. S.pyogenes is more sensitive to bacitracin than other streptococci. S.pyogenes can be rapidly identified by fluoruesent antibody technique. 4-Antigen detecting tests: ELISA, agglutination tests 5-Serological tests: test for streptococcal antibodies is not helpful in diagnosing acute infection which may be used to identify & confirm primary infection. They are more commonly used to diagnose non suppurative complications. Treatment S.pyogenes has not acquired resistance to penicillin G, which remains the antibiotic of choice for acute streptococcal disease. In a pencillin allergic patient , Macrolide such as clarithromycin is the preferred drug. Pencillin G plus clindamycin are used in treating streptococcal toxic shock syndrome. Group B β-hemolytic streptococci Group B streptococci, represented by the pathogen S.agalactiae. It is an important pathogen in neonates causing neonatal septicemia & meningitis. It is also associated with septic abortion & puerperial sepsis. S.agalactiae is a commensal of female genital tract from where bacterial colonization in neonates occur. Samples of blood, cervical swabs, spinal fluid can obtained for culture on blood agar. ELISA tests can also demonstrate the presence of bacterial antigen in these samples. Most isolates remain sensitive to penicillin G & ampicillin, which are still the antibiotics of choice. Enterococci Enterococci contain a C-carbohydrate that reacts with group D antisera. The clinically most important species are E. faecalis & E.faecium. Enterococci can be α-,β-, or non hemolytic. As a rule, enterococci are not very virulent, but they have become prominent as a cause of nosocomial infections as a result of their multiple antibiotic resistance. Epidemiology Enterococci are apart of normal flora. However, they can also colonize oral mucous membranes & skin, especially in hospital setting. Diseases Enterococci seldom cause disease in normal, healthy individuals. But under certain conditions, enterococci can spread to normally sterile sites, causing urinary tract infections (UTI), bacteremia sepsis, subacute bacterial endocarditis, biliary tract infection, or intra-abdominal abscesses. Non enterococcal group D streptococci Streptococcal bovis is the most clinically important of the non enterococcus group D streptococci. S.bovis occasionally causes UTI & subacute bacterial endocarditis. Viridans streptococci The viridans group of streptococci includes many gram- positive, catalse-negative, α- or γ-hemolytic species that constitute the main facultative oral flora. The viridans streptococci are relatively avirulent, but streptococcus mutans & other members of the viridans group cause dental caries. In patients with abnormal or damaged heart valves, they can also infect these valves during a bacteremia, causing subacute bacterial endocarditis. Streptococcus pneumoniae (pneumococcus) S. pneumoniae are gram positive, non motile, encapsulated cocci. They are lancetshaped, & their tendency to occur in pairs accounts for their earlier designation as diplococcus pneumoniae. Like other streptococci, S. pneumoniae is fastidious & routinely cultured on blood agar. It releases an α-hemolysin that damages red cell membranes, causing colonies to be αhemolytic. Epidemiology S. pneumoniae is an obligate parasite of humans, & can be found in the nasopharynx of many healthy individuals. This org is extremely sensitive to environmental agents pneumococcal infections can be either endogenous or exogenous. Pathogensis 1-Capsule: S. pneumoniae polysaccharide capsule is both antiphagocytic & antigenic. Antiphagocytic properties of the capsule protect the bacteria from polymorphonuclear leukocyte attack, facilitating growth of the bacteria prior to the appearance of anticapsular antibodies. There are approximately 85 distinct capsular serotypes, some of which endow strains with greater virulence than others, as reflected by the fact that about twenty serotypes account for vast majority of pneumococcal infections. 2-Autolysin: This peptidoglycan hydrolyse is present in the bacterial cell wall, & is normally inactive. However, it is readily triggered (ex, by surface active agents, β-lactam antibiotics, or aging), resulting in cell lysis. Autolysin is thus responsible for the release of intracellular virulence factors. 3- Pneumolysin: Although retained within the cytosol of intact pneumococci, pneumolysin is thought to be an important virulence factor by virtue of its ability to attack mammalian cell membranes, causing lysis once it is release by autolysin from the interior of the bacterium. 4-IgA protease: Pneumococci produce IgA protease that enhances the organism’s ability to colonize the mucosa of upper respiratory tract. Clinical significance Acute bacterial pneumonia: A leading cause of death, especially in the aged & those whose resistance is impaired, this disease is caused most frequently by S. pneumoniae . Otitis media Bacteremia/ sepsis Meningitis Lab diagnosis Specimens can be obtained from a nosapharyngeal swab, blood, pus, sputum, or spinal fluid. α-hemolytic colonies appear when S. pneumoniae is grown on the blood agar. Lancet-shaped, gram positive diplococci are observed on gram stain of the sample. Growth of these bacteria is inhibited by optochin , & the cells are lysed by bile acids. Capsular swelling is observed when the pneumococci treated with type-specific antisera (Quellung reaction).