carbohydrate structures
advertisement
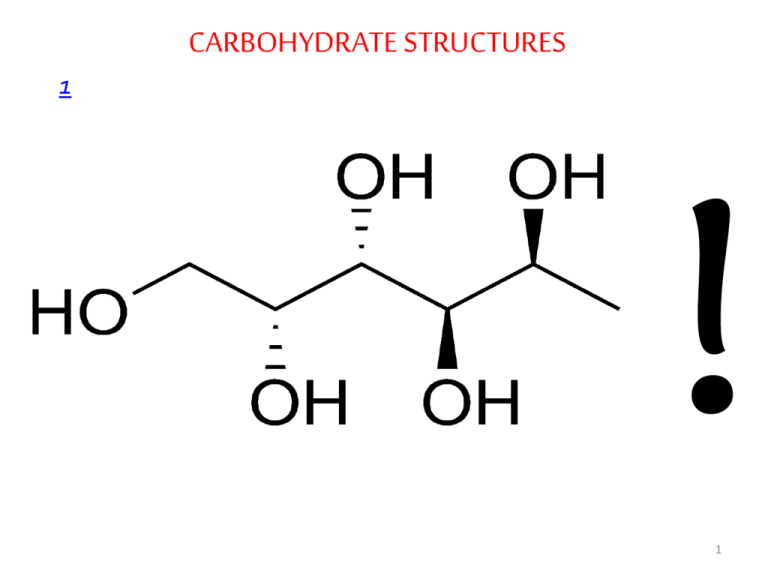
CARBOHYDRATE STRUCTURES 1 1 • • • • • Carbohydrates Carbohydrates, the most abundant biomolecules in nature, are a direct link between solar energy and the chemical energy of living systems The name carbohydrate arises from the basic molecular formula (CH2O)n, which can be rewritten (C.H2O)n to show that these substances are hydrates of carbon, where n=3 or more Carbohydrates constitute a versatile class of molecules. Energy from the sun captured by green plants, algae and some bacteria during photosynthesis is stored in the form of carbohydrates In turn, carbohydrates are the metabolic precursors of virtually all other biomolecules Glucose is the most important carbohydrate in humans ; most dietary carbohydrate is absorbed into the bloodstream as glucose formed by hydrolysis of dietary starch and disaccharides, and other sugars are converted to glucose in the 2 liver • In addition to being the major metabolic fuel, glucose is the precursor for synthesis of all the other carbohydrates in the body, including glycogen for storage; ribose and deoxyribose in nucleic acids; galactose in lactose of milk • Glucose and its derivatives are also major components of glycoconjugates • Three types of glycoconjugates –glycolipids, glycoproteins and proteoglycans – are important components of cell membranes, cell walls and extracellular structures in plants, animals, and bacteria • In addition to the structural roles that glycoconjugates play, investigations of biological processes such as signaltransduction, cell-cell interactions and endocytosis have revealed that they typically involve the binding of glycoproteins glycolipids, or free carbohydrate molecules with complementary receptors 3 carbohydrates have informational capacities • • • • • Carbohydrate Nomenclature Carbohydrates are generally classified into three groups: monosaccharides (and their derivatives), oligosaccharides, and polysaccharides The monosaccharides are also called simple sugars Oligosaccharides derive their name from the Greek word oligo, meaning “few,” and consist roughly of from two to twelve simple sugar molecules Disaccharides are common in nature, and trisaccharides also occur frequently. Four- to six-sugar-unit oligosaccharides are usually bound covalently to other molecules, including glycoproteins As their name suggests, polysaccharides are polymers of the simple sugars and their derivatives. They may be either linear or branched polymers and may contain hundreds or even thousands of monosaccharide units 4 Monosaccharides • Monosaccharides are aldehyde or ketone derivatives of polyhydroxy alcohols • Monosaccharides with an aldehyde functional group are called aldoses and those with a ketone group are called ketoses • The simplest aldose and ketose are glyceraldehyde and dihydroxyacetone, respectively • Simple sugars are also classified according to the number of carbon atoms they contain. The smallest sugars, called trioses, contain three carbon atoms; tetroses, pentoses, hexoses,… • The most abundant monosaccharides found in living cells are the pentoses and the hexoses • Simple sugars are usually represented by Fischer projections: the carbohydrate backbone is drawn vertically with the most highly oxidized carbon usually shown at the top; the horizontal lines are understood to project toward the viewer, and the 5 vertical lines recede from the viewer • • • • Stereochemistry of Monosaccharides Aldoses with at least three carbons and ketoses with at least four carbons contain chiral centers When the number of chiral carbons increases in optically active compounds, the number of possible optical isomers also increases The total number of isomers is given as 2n, where n is the number of chiral carbon atoms. Half of these isomers are going to be D isomers and the rest are L isomers The reference carbon is the asymmetric carbon that is farthest from the carbonyl carbon; its configuration is similar to that of 6 the asymmetric carbon in either D- or L- glyceraldehyde • Almost all naturally occurring sugars have the D-configuration just like L-amino acids • These preferences, established in apparently random choices early in evolution, persist uniformly in nature because of the stereospecificity of the enzymes that synthesize and metabolize these small molecules • In the D-aldose family of sugars, which contains most biologically important monosaccharides, the hydroxyl group is to the right of the chiral carbon atom farthest from the most oxidized carbon • The designation D or L merely relates the configuration of a given molecule to that of glyceraldehyde and does not specify the sign of rotation of plane-polarized light • Stereoisomers that are not enantiomers are called diastereomers • Diasteromers that differ in the configuration at a single 7 asymmetric carbon atom are called epimers Aldose Sugars 8 9 Ketose Sugars 10 11 • • • • • • Cyclic Structures and Anomeric Forms Although Fischer projections are useful for presenting the structures of particular monosaccharides and their stereoisomers, they don’t take into account the ability of sugars to form cyclic structures with formation of an additional asymmetric center Alcohols react readily with aldehydes to form hemiacetals The linear forms of glucose (and other aldohexoses) could undergo a similar intramolecular reaction to form a cyclic hemiacetal. The resulting six-membered, oxygen-containing ring is similar to pyran and is designated a pyranose In a similar manner, ketones can react with alcohols to form hemiketals The analogous intramolecular reaction of a ketose sugar such as fructose yields a cyclic hemiketal The five-membered ring thus formed is reminiscent of furan 12 and is referred to as a furanose Cyclic Aldoses 13 Cyclic Ketoses 14 Fischer Projection Formulas of Cyclic Glucose Fischer Projection Formulas of Cyclic Fructose 15 • The cyclic pyranose and furanose forms are the preferred structures for monosaccharides in aqueous solution • At equilibrium, the linear aldehyde or ketone structure is only a minor component of the mixture (generally much less than 1%) • When hemiacetals and hemiketals are formed, the carbon atom that carried the carbonyl function becomes an asymmetric carbon atom • Isomers of monosaccharides that differ only in their configuration about that carbon atom are called anomers, designated as α or β, and the carbonyl carbon is thus called the anomeric carbon • When the hydroxyl group at the anomeric carbon is on the same side of a Fischer projection as the oxygen atom at the highest numbered asymmetric carbon, the configuration at the anomeric carbon is α as in α-D-glucopyranose • When the anomeric hydroxyl is on the opposite side of the 16 Fischer projection, the configuration is β, as in β -D-glucopyranose • The anomers of monosaccharides are readily interconverted when dissolved in water • This spontaneous process, called mutatrotation, produces a mixture of α and β forms in both furanose and pyranose ring structures • This process occurs more rapidly in the presence of cellular enzymes called mutarotases • The proportion of each form differs with each sugar type • The open chain formed during mutarotation can particpate in oxidation-reduction reactions • Although Haworth projections are convenient for display of monosaccharide structures, they do not accurately portray the conformations of pyranose and furanose rings • Neither pyranose nor furanose rings can adopt true planar structures. Instead, they take on puckered conformations, and in the case of pyranose rings, the two favored structures are the 17 chair conformation and the boat conformation Chair and Boat Conformations of Pyranose Sugars 18 Important Monosaccharides • Among the most important monosaccharides that occur in living things are glucose, fructose and galactose Glucose • D-glucose, originally known as dextrose is the primary energy source for living cells • It is the preferred energy source for brain cells and cells with little or no mitochondria such as erythrocytes; and for cells with limited supply of oxygen such as the eyeball and the renal medulla Fructose • D-fructose, originally called levulose, is often referred to as fruit sugar because of its high content in fruit • It is synthesized in the seminal vesicles and then incorporated into semen. Sperm use the sugar as an energy source Galactose • Galactose is involved in the synthesis of biomolecules such as 19 lactose, glycolipids, glycoproteins and proteoglycans • Synthesis of these substances is not diminished by diets that lack galactose or its main dietary source, lactose, because galactose can be synthesized from glucose by an epimerase Derivatives of Monosaccharides • A variety of chemical and enzymatic reactions produce derivatives of the simple sugars Sugar Acids • Sugars with free anomeric carbon atoms are reasonably good reducing agents and will reduce hydrogen peroxide, certain metals (e.g. Cu2+ ) and other oxidizing agents.Such reactions convert the sugar to a sugar acid • For example, addition of alkaline CuSO4 (called Fehling’s solution) to an aldose sugar produces a red cuprous oxide (Cu2O) precipitate: 20 • Carbohydrates that can reduce oxidizing agents in this way are referred to as reducing sugars • Sugars whose aldehyde group has been oxidized are known as aldonic acids such as gluconic acid • If the carbon containing the terminal hydroxyl group is oxidized, the sugar is called a uronic acid (e.g., glucuronic acid) Sugar Alcohols • Sugar alcohols, or alditols, are designated by the addition of -itol to the name of the parent sugar. The alditols are mostly 21 linear molecules that cannot cyclize in the manner of aldoses • Sugar alcohols are not metabolized as rapidly as their precursors, and reconversion to the precursor is also slow • Alditols are characteristically sweet -tasting, and sorbitol (D-glucitol), mannitol and xylitol are widely used to sweeten sugar-free gums • Sorbitol buildup in the eyes is implicated in cataract formation caused by diabetes and galactosemia • Clinically, mannitol is administered intravenously as an osmotic diuretic in patients with acute renal failure. It is not metabolized appreciably, is filtered by the glomerulus, and is not reabsorbed by the tubules; hence it is excreted in urine • The non-reabsorbable solute holds water and thus maintains urine volume in the presence of decreased glomerular function • Intravenous mannitol is also used to relieve an increase in pressure and in volume of cerebrospinal fluid • Glycerol and myo-inositol, a cyclic alcohol, are components of 22 lipids 23 Deoxy Sugars • The deoxy sugars are monosaccharides with one or more hydroxyl groups replaced by hydrogens • 2-Deoxy-D-ribose whose systematic name is 2-deoxy-Derythropentose, is a constituent of DNA in all living things • Deoxy sugars also occur frequently in glycoproteins and polysaccharides • L-Fucose and L-rhamnose, both 6-deoxy sugars, are components of some cell walls, and rhamnose is a component of ouabain, a highly toxic cardiac glycoside found in the bark and root of the ouabaio tree • L-Fucose is often found among the carbohydrate components of glycoproteins, such as those of the ABO blood group determinants on the surface of red blood cells 24 25 Amino sugars • Amino sugars are obtained by replacing a hydroxyl group of a monosaccharide by an amino group • The most common amino sugars are the 2aminoaldohexoses, namely, D-glucosamine and Dglucosamine and D-galactosamine • The amino groups usually occur as N-acetyl derivatives • Amino sugars are components of structural polysaccharides and of glycolipids of membranes • N-Acetyl muramic acid , a constituent of a bacterial cell wall polysaccharide (hence named as an amine isolated from bacterial cell wall polysaccharides; murus is Latin for “wall”), has a lactyl side chain linked to C3 of glucosamine • The polysaccharide of bacterial cell walls is a polymer of alternating N-acetylmuramic acid and N-acetyl26 glucosamine • N-Acetylneuraminic acid, NANA (an amine isolated from neural tissue) is made from the linkage of Nacetylmannosamine with pyruvic acid • The N-acetyl and N-glycolyl derivatives of neuraminic acid are collectively known as sialic acids and are distributed widely in membrane systems in bacteria and animals 27 Glycosides • The hydroxyl group on the anomeric carbon of a monosaccharide can react with an –OH or an –NH group of another compound to form a glycosidic bond • When the second compound is not another monosaccharide, it is known as an aglycone • be another monosaccharide • These bonds are known as, respectively, O-glycosidic and Nglycosidic bonds • The linkage may be either α or β , depending on the position of the atom attached to the anomeric carbon of the sugar • N-glycosidic bonds are found in nucleosides, nucleotides and glycoproteins • For example, in the adenosine moiety of ATP, the nitrogenous base adenine is linked to the sugar ribose through a β -N28 glycosidic bond • In contrast, O-glycosidic bonds join sugars to each other or attach sugars to the hydroxyl group of an amino acid on a protein • The glycosides that are important in medicine because of their action on the heart (cardiac glycosides) all contain steroids as the aglycone • These include digitalis and ouabain, which are inhibitors of the Na+-K+ ATPase of cell membranes. Other glycosides include antibiotics such as streptomycin. 29 Oligosaccharides • The simplest oligosaccharides , disaccharides, consist of two monosaccharide units linked by an O-glycosidic bond • As in proteins ,each individual unit in an oligosaccharide is termed a residue • The disaccharides that are most commonly found in nature and are of physiologically importance are sucrose, maltose and lactose • The oxidation of a sugar’s anomeric carbon by cupric or ferric ion occurs only with the linear form, which exists in equilibrium with the cyclic form(s) • When the anomeric carbon is involved in a glycosidic bond, that sugar residue cannot take the linear form and therefore becomes a non-reducing sugar • In describing disaccharides or polysaccharides, the end of a chain with a free anomeric carbon (one not involved in a glycosidic 30 bond) is commonly called the reducing end • Maltose, isomaltose,cellobiose and trehalose are all homodisaccharides because they each contain only one kind of monosaccharide, namely, glucose • Maltose is a is a component of malt, a substance obtained substance obtained by allowing grain to soften in water and germinate • Maltose contains two D-glucose residues joined by a glycosidic linkage between C-1 (the anomeric carbon) of one glucose residue and C-4 of the other • Because it retains a free anomeric carbon, maltose is a reducing sugar. The linkage in maltose is represented as (α1 → 4) and maltose could be written as Glc (α1 → 4) Glc • Cellobiose is Glc (β1 → 4)Glc, isomaltose Glc (α 1 → 6)Glc and trehalose Glc (α1 → 1 α) Glc • Lactose, which yields D-galactose and D-glucose on hydrolysis, occurs naturally only in milk 31 Common Disaccharides 32 • The anomeric carbon of the glucose residue is available for oxidation, and thus lactose is a reducing disaccharide • Lactose’s abbreviated name is Gal (β1 → 4)Glc • Sucrose (table sugar) is a disaccharide of glucose and fructose. It is formed by plants but not by animals • In contrast to maltose and lactose, sucrose contains no free anomeric carbon atom; the anomeric carbons of both monosaccharide units are involved in the glycosidic bond. Sucrose is therefore a nonreducing sugar • Sucrose can be represented either as Glc (α 1 → 2 β) Fru or Fru (β2 → 1α) Glc Stachyose is typical of the oligosaccharide components found in substantial quantities in beans, peas, bran and whole grains These oligosaccharides are not digested by stomach enzymes, but are metabolized readily by bacteria in the intestines. This is the source of the flatulence that often accompanies the consumption 33 of such foods • • • • • Polysaccharides The majority of carbohydrate material in nature occurs in the form of polysaccharides Polysaccharides, also called glycans, consist of monosaccharides and their derivatives If a polysaccharide contains only one kind of monosaccharide molecule, it is a homopolysaccharide, or homoglycan, whereas those containing more than one kind of monosaccharide are heteropolysaccharides The most common constituent of polysaccharides is D-glucose, but D-fructose, D-galactose, L-galactose, D-mannose, Larabinose, and D-xylose are also common Common monosaccharide derivatives in polysaccharides include the amino sugars (D-glucosamine and D-galactosamine), their derivatives (N-acetylneuraminic acid and N-acetylmuramic acid), and simple sugar acids (glucuronic and 34 iduronic acids) • Based on their functions, polysaccharides have been traditionally classified into storage materials, structural components or protective substances • An improvement on this classification is the discovery that polysaccharides and oligosaccharides function as information carriers: they serve as destination labels for some proteins and as mediators of specific cell-cell interactions and interactions between cells and the extracellular matrix Storage Polysaccharides • The most important storage polysaccharides are starch in plant cells and glycogen in animal cells • Both polysaccharides occur intracellularly as large clusters or granules • Starch and glycogen molecules are heavily hydrated, because they have many exposed hydroxyl groups available to hydrogen-bond with water 35 • Starch contains two types of glucose polymer, amylose and amylopectin • Amylose consists of long, unbranched chains of D-glucose residues connected by (α1 → 4) linkages. Such chains vary in molecular weight from a few thousand to more than a million • Unlike proteins, polysaccharides generally do not have definite molecular weights. This is because proteins are synthesized on a template (messenger RNA) of defined sequence and length • Amylopectin also has a high molecular weight (up to 100 million) but unlike amylose is highly branched • The glycosidic linkages joining successive glucose residues in amylopectin chains are (α1 → 4); the branch points (occurring every 24 to 30 residues) are (α1 → 6) linkages • Glycogen is found mainly in the liver (where it may amount to as much as 10% of liver mass) and skeletal muscle (where it accounts for 1 to 2% of muscle mass) 36 • Like amylopectin, glycogen is a polymer of (α1 → 4) -linked subunits of glucose, with (α1 → 6) -linked branches, but glycogen is more extensively branched (on average, every 8 to 12 residues) and more compact than starch • In hepatocytes glycogen is found in large granules, which are themselves clusters of smaller granules composed of single, highly branched glycogen molecules with an average molecular weight of several million • Because each branch in glycogen (and also amylopectin) ends with a non-reducing sugar unit, a glycogen molecule has as many non-reducing ends as it has branches, but only one reducing end • Storing glucose in the form of glycogen significantly reduces the osmolality that the cell would have had if glucose was stored in free form the threat of osmotic lysis is averted 37 Storage Polysaccharides 38 • Dextrans are bacterial and yeast polysaccharides made up of (α1→6)-linked poly-D-glucose; all have (α1→3)branches, and some also have (α1→2) or (α1→4) branches • Dental plaque, formed by bacteria growing on the surface of teeth, is rich in dextrans • Inulin is a polysaccharide of fructose. It is readily soluble in water and is used to determine the glomerular filtration rate, but it is not hydrolyzed by intestinal enzymes Structural Polysaccharides • The structural polysaccharides have properties that are dramatically different from those of the storage polysaccharides, even though the compositions of these two classes are similar • The structural polysaccharide cellulose is the most abundant natural polymer found in the world • Found in the cell walls of nearly all plants, cellulose is one of the 39 principal components providing physical structure and strength • Like amylose, the cellulose molecule is a linear, unbranched homopolysaccharide, consisting of 10,000 to 15,000 D-glucose units • But there is a very important difference: in cellulose the glucose residues have the β-configuration • The glucose residues in cellulose are linked by (β 1→4) glycosidic bonds • This linkage leads to cellulose assuming a fully-extended conformation that permits efficient interchain hydrogen bonding, the basis of much of the strength of cellulose • The water content of cellulose structure is low because extensive interchain hydrogen bonding between cellulose molecules satisfies their capacity for hydrogen-bond formation • Chitin is a linear homopolysaccharide composed of N-acetylglucosamine residues in β-linkage • The only chemical difference from cellulose is the replacement of the hydroxyl group at C-2 with an acetylated amino group40 • Chitin forms extended fibers similar to those of cellulose • Chitin is the principal component of the hard exoskeletons of nearly a million species of arthropods—insects, lobsters, and crabs, for example— and is probably the second most abundant polysaccharide in nature 41 • Chitin forms extended fibers similar to those of cellulose • Chitin is the principal component of the hard exoskeletons of nearly a million species of arthropods—insects, lobsters, and crabs, for example— and is probably the second most abundant polysaccharide in nature • The rigid component of bacterial cell walls is a heteropolymer of alternating (β 1→4) -linked N-acetylglucosamine and Nacetyl-muramic acid residues • The polysaccharide chains are cross-linked through short peptide chains, and the overall structure is known as a peptidoglycan • The peptides are composed of both D and L amino acids and they weld the polysaccharide chains into a strong sheath that envelops the entire cell and prevents cellular swelling and lysis due to the osmotic entry of water • The enzyme lysozyme (also called muramidase) kills bacteria by 42 hydrolyzing the (β 1→4) bond The Structure of Peptidoglycan • Penicillin and related antibiotics kill bacteria by preventing synthesis of the cross-links, leaving the cell wall too weak to resist osmotic lysis 43 • • • • • Protective Polysaccharides The extracellular space in the tissues of multicellular animals is filled with a gel-like material, the extracellular matrix (ECM), also called ground substance, which holds the cells together and provides a porous pathway for the diffusion of nutrients and oxygen to individual cells The ECM is composed of an interlocking meshwork of heteropolysaccharides and fibrous proteins such as collagen, elastin, fibronectin and laminin These heteropolysaccharides, the glycosaminoglycans, are a family of linear polymers composed of repeating disaccharide units One of the two monosaccharides is always either N-acetylglucosamine or N-acetylgalactosamine; the other is in most cases a uronic acid, usually D-glucuronic or L-iduronic acid In some glycosaminoglycans, one or more of the hydroxyls of 44 the amino sugar are esterified with sulfate • The combination of of sulfate groups and the carboxylate groups of the uronic acid residues gives glycosaminoglycans a very high density of negative charge • To minimize repulsion between neighboring charged groups, these molecules assume an extended conformation in solution • Heparin, with the highest net negative charge of the disaccharides shown, is a natural anticoagulant substance. It binds strongly to antithrombin III and inhibits blood clotting • Hyaluronates are important components of the vitreous humor in the eye and of synovial fluid, the lubricant fluid of joints in the body • The chondroitins and keratan sulfate are found in tendons, cartilage, and other connective tissue, whereas dermatan sulfate, as its name implies, is a component of the extracellular matrix of skin • Glycosaminoglycans (except hyaluronate),in combination with 45 proteins, give rise to proteoglycans 46 Carbohydrates as Informational Molecules: The Sugar Code • Cells use specific oligosaccharides located on glycoproteins and glycolipids to encode important information about intracellular targeting of proteins, cell-cell interaction, tissue development and extracellular signals • Branched structures, not found in nucleic acids or proteins, are common in oligosaccharides. These means that more information can be encoded by an oligosaccharide that contains the same number of residues as a protein or a nucleic acid 47 • The sugar code is “read” by lectins: lectins, found in all organisms, are proteins that bind carbohydrates with high affinity and specificity • Biological processes that involve lectin binding include a vast array of cell-cell interactions • Prominent examples include infections by microorganisms, the mechanisms of many toxins and physiological processes such as leukocyte rolling Infections • Infection by many bacteria is initiated when they become firmly attached to host cells • Often, attachment is mediated by the binding of bacterial lectins to oligossacharides on the cell’s surface • Helicobacter pylori ,a major causative agent of gastritis and stomach ulcers, possesses several lectins that allow it to establish a chronic infection in the mucous membrane of the 48 stomach • One of these lectins binds with high affinity to a portion of the type O blood group determinant, an oligosaccharide, a circumstance that explains the observation that humans with type O blood are at considerably greater risk of developing ulcers • Several animal viruses, including the influenza virus, attach to their host cells through interactions with oligosaccharides displayed on the host cell surfaces • The lectin of the influenza virus, the HA protein, is essential for viral entry and infection. After initial binding of the virus to a sialic acid–containing oligosaccharide on the host surface, a viral sialidase removes the terminal sialic acid residue, triggering the entry of the virus into the cell. Inhibitors of this enzyme are used clinically in the treatment of influenza Entry of toxins into the cell • The damaging effects of many bacterial toxins occur only after endocytosis into the host cell, a process that is initiated by lectinligand binding 49 • The binding of the B subunit of cholera toxin to a glycolipid on the surface of intestinal cells results in the uptake of toxic A subunit • Once the A subunit is internalized, it proceeds to disrupt the mechanism that regulates chloride transport, a process that results in a life-threatening diarrhea Leukocyte rolling • When a tissue becomes damaged in an animal either by infection or physical trauma, it emits signal molecules that create an inflammation • In response to certain of these molecules, the endothelial cells that line nearby blood vessels produce and insert a protein known as selectin into their plasma membranes • The selectins are a family of lectins that act as cell-adhesion molecules. Once selectin is displayed on the surface of the endothelial cells, it binds transiently to its ligand oligosaccharides on white blood cells such as neutrophils 50 • This relatively weak binding serves to slow the rapid motion of neutrophils as they flow in blood so that they appear to roll along the lumenal surface of the blood vessel • Once rolling has been initiated, and white blood cells approach the inflammation site, they encounter other signal molecules that cause them to express another lectin called integrin on their surfaces • The binding of integrin with its oligosaccharide ligand on the endothelial surface of the blood vessel causes the WBC to roll • Subsequently, the WBC undergo changes that allow them to squeeze between the cells of the endothelium and migrate to the infected where they proceed to consumed and degrade bacteria and cellular debris Normal physiological processes mediated by oligosaccharidelectin interactions include protein targeting to cellular compartments and hepatic degradation of certain peptide 51 hormones 52