Chem 11 HL – Quiz 2
advertisement
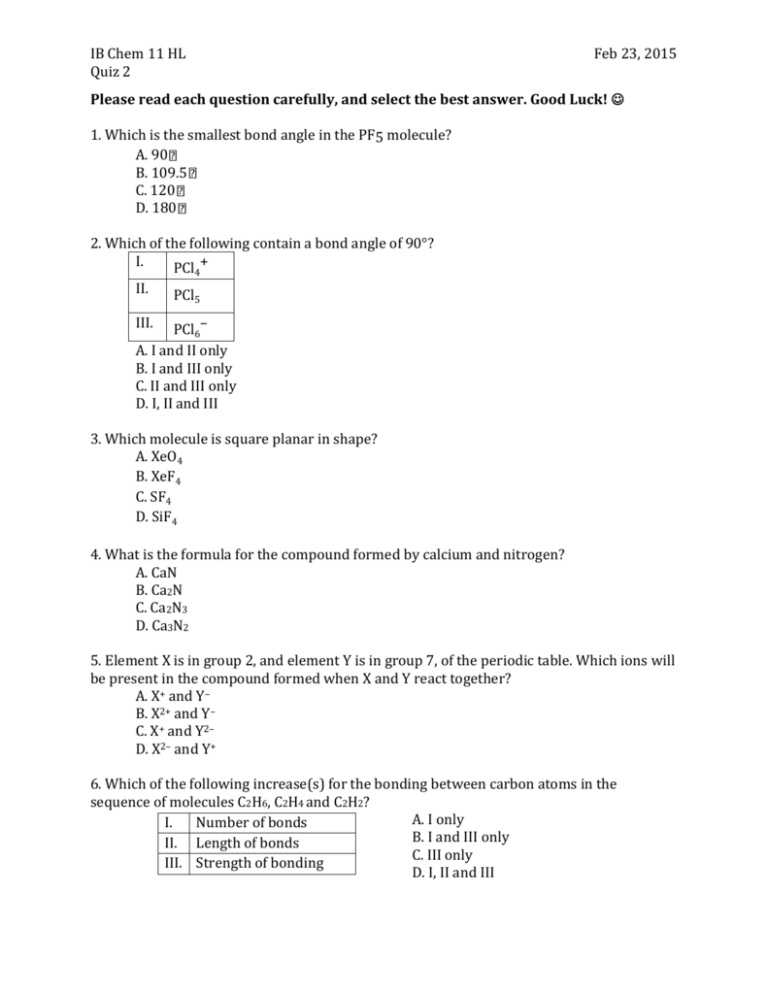
IB Chem 11 HL Quiz 2 Feb 23, 2015 Please read each question carefully, and select the best answer. Good Luck! 1. Which is the smallest bond angle in the PF5 molecule? A. 90 B. 109.5 C. 120 D. 180 2. Which of the following contain a bond angle of 90°? I. PCl + 4 II. PCl5 III. PCl6– A. I and II only B. I and III only C. II and III only D. I, II and III 3. Which molecule is square planar in shape? A. XeO4 B. XeF4 C. SF4 D. SiF4 4. What is the formula for the compound formed by calcium and nitrogen? A. CaN B. Ca2N C. Ca2N3 D. Ca3N2 5. Element X is in group 2, and element Y is in group 7, of the periodic table. Which ions will be present in the compound formed when X and Y react together? A. X+ and Y– B. X2+ and Y– C. X+ and Y2– D. X2– and Y+ 6. Which of the following increase(s) for the bonding between carbon atoms in the sequence of molecules C2H6, C2H4 and C2H2? A. I only I. Number of bonds B. I and III only II. Length of bonds C. III only III. Strength of bonding D. I, II and III IB Chem 11 HL Quiz 2 Feb 23, 2015 7. What is the Lewis (electron dot) structure for sulphur dioxide? A. B. C. D. 8. Which substance is most similar in shape to NH3? A. GaI3 B. BF3 C. FeCl3 D. PBr3 9. Which statement is a correct description of electron loss in this reaction? 2Al + 3S Al2S3 A. Each aluminium atom loses two electrons. B. Each aluminium atom loses three electrons. C. Each sulphur atom loses two electrons. D. Each sulphur atom loses three electrons. 10. Which compound contains both ionic and covalent bonds? A. MgCl2 B. HCl C. H2CO D. NH4Cl 11. Which statement is true for compounds containing only covalent bonds? A. They are held together by electrostatic forces of attraction between oppositely charged ions. B. They are made up of metal elements only. C. They are made up of a metal from the far left of the periodic table and a non-metal from the far right of the periodic table. D. They are made up of non-metal elements only. 12. Which substance has the lowest electrical conductivity? A. Cu(s) B. Hg(l) C. H2(g) D. LiOH(aq) IB Chem 11 HL Quiz 2 Quiz 2 – Answer Key 1. A 2. C 3. B 4. D 5. B 6. B 7. D 8. D 9. B 10. D 11. D 12. C Feb 23, 2015