ChangesofState3_2
advertisement
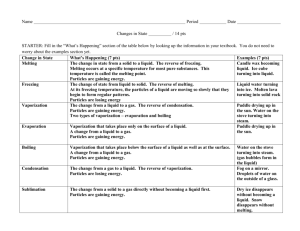
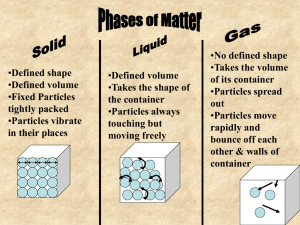
•What happens to a puddle of water on a warm day? •What happens to ice if left outside on a warm day? •What happens to a pond during very cold temperatures? •States of matter depend on molecular motion. •Matter changes states when thermal energy is added or taken away. •(((O)))(((O)))= O O •Solids become a liquid by getting so much energy they move faster and faster then are broken free from their fixed position. •When a solid changes to a liquid we call this MELTING. •How do we know when a solid gets too much energy and will break free from their fixed position? •Melting point- a certain temperature that will cause a solid’s particles to break free. •SOLID TO LIQUID= MELTING The opposite of melting is ____________? •Freezing- the change from a liquid to a solid. •When a substance freezes the particles in a liquid move so slowly that they begin to take on fixed position, freezing. Or becoming a solid. •In order to make something freeze, or become a solid you must take away ENERGY. How do you do this? By placing a substance into a freezer, and the freezer slows down the particles by taking away energy and turning liquids into solids. •Melting and freezing are reversible, and are NOT chemical changes. Vaporization- takes place when the particles in a liquid gain enough energy to move independently, forming a gas. Two types of Vaporization 1. Evaporation- when a liquid vaporizes only on the top surface of a liquid. 2. Boiling- when a liquid changes into a gas from below and on the surface. • Boiling Point- the temperature at which a liquid boils. Sublimation- occurs when surface particles of a solid gain enough energy that they form a gas. •During sublimation particles of a solid do not pass through the liquid state as they form a gas. •As dry ice changes states it absorbs thermal energy. •Typically this occurs in colder climates where there is snow, and usually the temperature will remain below 0 Celsius but still lose snow from the ground. •How does the motion of the particles change during sublimation? http://teacher.scholastic.com/activities /studyjams/matter_states/ Energy Melting ((0)) ((0)) ((0)) particles go crazy Energy Vaporization O O particles go crazy