File - chohan`s chemistry
advertisement

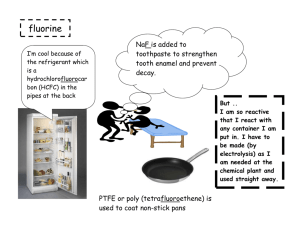
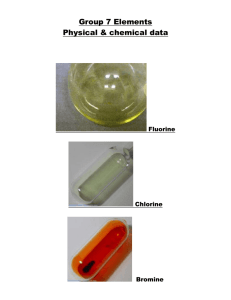
Group 7 – the halogens The elements in group 7 of the periodic table, on the right, are called the halogens. F fluorine Cl chlorine Br bromine I At iodine astatine • Halogens form ions with a -1 charge • Halides (e.g. Cl-) are negative ions which are made when halogen atoms gain an electron. This happens when the halogens make compounds (e.g. NaCl, KBr). • Halogens all exist as DIATOMIC MOLECULES (i.e. F2, Cl2, Br2, I2) – see next slide • Halogens are all coloured elements All the Group 7 elements are molecules containing two atoms. (They are diatomic) Each atom is 1 electron short of a noble gas electron structure. By sharing electrons in a covalent bond full outer electron shells are achieved. F F F F Halogens – what do they look like? Chlorine Bromine Iodine Element Colour Size Melting Point (oC) Boiling Point (oC) Physical State Fluorine Yellow -220 -188 GAS Chlorine Green -101 -35 GAS Bromine Orangebrown -7 59 LIQUID Iodine Purple +114 184 SOLID Fluorine Chlorine Bromine Iodine As you go down the group: • The halogens become darker in colour • They become denser • The melting and boiling point increases • The radius of the atom increases because there are more electron shells • The elements become LESS reactive Halogen vapours Bromine and iodine are not gaseous, but have low boiling points. This means that they produce vapour at relatively low temperature. They are volatile. Bromine produces some orange-brown vapour, seen here above, the vapour diffuses up the gas jar. When iodine is heated gently, it changes directly from a solid to a gas without first becoming a liquid. This is called sublimation. All the Group 7 elements have 7 electrons in the outermost shell. F Cl Chlorine Br Bromine I At 2,7 Fluorine Iodine 2,8,7 And so on Astatine So, when group 7 elements react they need to GAIN an electron to form a 1- ion. BECOME LESS REACTIVE WHY DO THE HALOGENS BECOME LESS REACTIVE GOING DOWN THE GROUP? As we go down the group: The size of the atom increases because there are more electron shells So the outer shell electrons are further away from the positive nucleus, so when an electron is added to the halogen it is less attracted to the positive nucleus Also there is more shielding of inner shells so again the electron is less attracted to the nucleus and so the halogens become less reactive Reactivity of fluorine • The smaller the halogen atom the more strongly it attracts an electron to form a halide ion. Fluorine is more reactive than the other halogens HALOGEN DISPLACEMENT REACTIONS A less reactive halogen can be displaced by a less reactive halogen from solution. The organic solvent which dissolves the halogen is called CYCLOHEXANE Cyclohexane dissolves the halogen so that we can see the colour of the halogen dissolved SUMMARY OF DISPLACMENT REACTIONS Halogen solution Halogen Potassium Potassium chloride bromide Chlorine x Bromine x √ x Iodine x x X = reactions which do not work √ = reaction which do work Potassium iodide √ √ x SUMMARY OF OBSEVATIONS MADE IN THE CYCLOHEXANE Halogen solution Halogen Chlorine Potassium Potassium chloride bromide x Orange/brown colour seen Bromine x x Iodine x x Potassium iodide Purple colour seen Purple colour seen x Halogen displacement reactions Halogen displacement reactions are redox reactions. The equations below show the reactions that did work CHLORINE + POTASSIUM BROMIDE POTASSIUM CHLORIDE + BROMINE Cl2 + 2KBr 2KCl + Br2 An orange brown colour is seen in the test tube because bromine has been displaced The half equations for this reactions are: Cl2 + 2e- 2Cl2Br- Br2 (this is a reduction as electrons are gained) (this is an oxidation as electrons are lost) CHLORINE + POTASSIUM IODIDE POTASSIUM CHLORIDE + IODINE Cl2 + 2KI 2KCl + I2 The half equations for this reactions are: Cl2 + 2e- 2Cl2I- I2 (this is a reduction as electrons are gained (this is an oxidation as electrons are lost) A purple colour is seen in the test tube because iodine is displaced BROMINE + POTASSIUM IODIDE POTASSIUM BROMIDE + IODINE Br2 + 2KI 2KBr + I2 A purple colour is seen in the test tube since iodine has been displaced The half equations for this reactions are: Br2 + 2e- 2Br2I- I2 (this is a reduction as electrons are gained (this is an oxidation as electrons are lost) IONIC EQUATIONS FOR DISPLACEMENT REACTIONS The ionic equations for the reactions that did work are shown below: Cl2 + 2Br- 2Cl- + Br2 Cl2 + 2I- 2Cl- + I2 Br2 + 2I- Br- + I2 http://chemstuff.co.uk/2012/12/21/displacement-reactions/ The video above shows a displacement reaction carried out in a laboratory BONDING IN HALOGEN COMPOUNDS Halogens can form both ionic and covalent compounds. 1. REACTION OF HALOGENS WITH SODIUM SODIUM + HALOGEN SODIUM HALIDE EXAMPLES Sodium + Chlorine Sodium chloride (2Na(s) + Cl2(g) 2NaCl) Sodium + Bromine Sodium bromide (2Na(s) + Br2(l) 2NaBr(s)) Sodium + Iodine Sodium iodide (2Na(s) + I2(g) 2NaI) This compound is ionic and therefore has strong electrostatic forces of attraction between opposite ions i.e. strong ionic bonds Ionic Bonding In NaCl Na Cl - + Na Cl 2. REACTIONS WITH HYDROGEN GAS The halogens react with hydrogen gas to product hydrogen halides. HYDROGEN + HALOGEN HYDROGEN HALIDE REACTIONS BECOME LESS VIGOROUS GOING DOWN THE GROUP Fluorine explosive even at -2000C and in the dark Chlorine and hydrogen explode in bright sunlight but react slowly in the dark. Bromine and hydrogen react slowly on heating with a platinum catalyst. Iodine combines partially and very slowly with hydrogen, even on heating with platinum catalyst. Hydrogen halides are covalently bonded compounds. Covalent compounds SHARE electrons: H Cl