Rahwa Netsanet 9/29/09 Osmosis and Diffusion Lab Report
advertisement

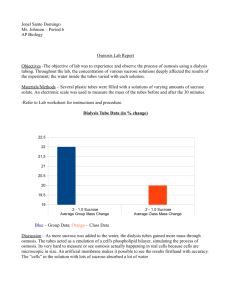
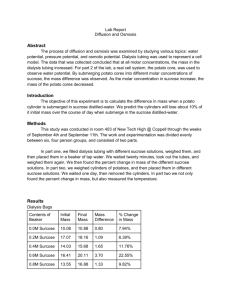
Rahwa Netsanet 9/29/09 Osmosis and Diffusion Lab Report Osmosis: movement of water down its concentration gradient, from high concentration to low concentration. Diffusion: movement of substances, other than water, down their concentration gradient, from high concentration to low concentration. Processing Raw Data: Part A Potato Core Individual Data: Contents in Beaker f) 1.0M Sucrose Initial Mass Final Mass Mass Difference % Change in Mass 10.1g 7g 3.1g 30.7% Percent Change in Mass = Final Mass - Initial Mass Initial Mass X 100 Potato Core Results, Class Data: Percent Change in Mass of Potato Cores (n) M Sucrose Group 1 (%) Distilled 13.8 Water 0.2 M 5.66 0.4 M 6.38 0.6 M -21.8 0.8 M -25.9 1.0 M -17.9 X= not available (Graph Turned In) Class Average (%) Expected Percentage (%) X 6th Period Average (%) 13.6 15.0 21.4 X X X X -30.7 10.5 1.85 -22.4 -23.35 -31.2 8.6 5.8 -22.0 -24.6 -26.8 6.9 -4.5 -12.8 -23.5 -23.5 Group 2 (%) Group 3 (%) Group 4 (%) Group 5 (%) 18.8 20 7.2 7.55 12.96 -21.6 -25.9 -22.4 11.1 3.7 -30.9 -21.2 -32.7 9.89 0 -13.9 -25.5 -30.2 Part B Visual from Magnified Cell through Microscope(shown as group visual on left, expected visual on right): Slide 1: Small amount of water added: (visuals turned in) Slide 2: Small amount of salt added: (visuals turned in) Slide 3: Small amount of water added again: (visuals turned in) Part C Dialysis Bag Individual Data: Contents in Dialysis Bag f) 1.0 M Sucrose Initial Mass Final Mass Mass Difference % Change in Mass 25g 30g 5g 20% Dialysis Bag Results, Class Data: Percent Change in Mass of Dialysis Bags (n)M Sucrose In Bag Group 1 (%) Distilled -4.16 Water 0.2 M 0 0.4 M 7.69 0.6 M 9.09 0.8 M 10.78 1.0 M 14.82 X= not available (Graph Turned In) Class Average (%) Expected Percentage (%) X 6th Period Average (%) -0.61 -1.35 1.2 X X X X 20 2.97 4.87 11.7 16 13.4 2.48 6.355 9.2 13.47 13.52 3.1 7.7 11.0 14.8 18.2 Group 2 (%) Group 3 (%) Group 4 (%) Group 5 (%) 0 0 -1.22 4 8 6.8 11.1 19.05 4.4 8.63 11.7 16 8.21 1.53 1.1 X 16 12 Conclusion and Evaluation: Part A: Concluding: It can be concluded that the more saturated a solution is with sucrose, the more negative the percent change in mass of the potato cores becomes. Osmosis occurred in Part A, where potato cores were soaked in a solution for a period of time. Water undergoes osmosis, where it goes down its concentration gradient into the potato cores, where there is less water per unit square than outside the potato cores. When a potato core was soaked in distilled water, its mass increased by the expected value of 21.4%. When the solution has 0.2 M of sucrose added, then the percent change decreases to 6.9%. At 0.6 M of sucrose, the expected value for percent change is -12.8%, and for 1.0 M Sucrose, the expected value for the change in mass is -23.5%. The more saturated with sucrose the solution is, the more negative the potato core’s expected percent change in mass becomes. This corresponds with the fact that potatoes have sucrose in them, but little water. Considering the distilled water solution, the water would diffuse into the potato, down its concentration gradient, because there is little water inside the potato and the water molecules are small enough to diffuse into cell membrane of the potato. This trend is also observed in the Class Average and 6th Period Average columns. Sucrose is a larger molecule, therefore it weighs much more than water in grams: one sucrose molecule is 294.31g, one water molecule is 18g, when there are 6.022 x 1023 molecules of both substances. In this lab, fractions of these STP values for the molecules’ weights are in action. But this makes it very difficult for sucrose to move out of the potato cores into the solution. In general, the potato cores hold onto all the sucrose molecules within them, so when water diffuses into the cell membranes of the potato cores, the weight of the water is added to the weight of the sucrose already there. The less water there is available for diffusion, i.e. a more saturated concentration of sucrose there is, the less water diffuses into the potato, making it weigh less than solutions with more water concentration. In the majority of the cases, the potato lost mass, giving a negative percent of change. This occurs when water diffuses out of the potato, down its concentration gradient. A more negative percent of change is correlated with a higher concentration of sucrose in the solution the potato cores are soaked in, meaning that when water is in low concentration in the solution, the water within the potato cores diffuses out to evenly distribute itself by moving down its concentration gradient from high concentration to low concentration. It can be concluded then that at higher concentration of sucrose in the solution, the more negative the percent change of mass of the potatoes is because water moves down its concentration gradient from high to low concentration. Evaluating Procedure: One weakness of this lab was that the potatoes were older, meaning they may have had more sucrose stored than younger potatoes would, changing the data a bit. Limitations in this lab consist of shared, exchanged and moved materials. The materials used in this lab were the same in each biology class, meaning many other people used the materials period 6 used, making it possible that contamination took place from other materials or from the people using the materials. Materials were also exchanged between people during the lab, such as beakers, pipettes and paper towels, making it more possible that particular samples were contaminated by the substance another group was testing. Moving materials was also a problem. Since so many people needed certain materials at certain times, some materials such as flasks, empty beakers and solution-filled beakers and plastic covers, were moved around to accommodate people’s needs who could not reach over others to get these materials or were crowded out and needed space to continue setting up and doing the lab. There is also the odd Group 5 set number, which somewhat throws off the information. It is unclear whether this group was foreknown or if the addition of the data was an accident. The limited amount of materials, the old potatoes used and the extra number under Group 5 for 1.0 M Sucrose contribute to the inconsistent numbers between classes which conducted the lab. Improving the Investigation: A simple way to make sure old potatoes are not used would be to buy fresh potatoes the day the lab begins, so that the data would not be skewed by a small increase of sugar in the potatoes. To improve the limitations on materials, cleanliness would have to be an issue. Though materials for each individual person would be optimal, cleaning the materials used in class would be helpful to the next class. A cleaner would have to be used that would not obstruct the lab, so that the following class does not receive any false data. Exchanging or moving materials should be prevented, so that materials do not get mislabeled or lost, and do not get contaminated by other substances. In regards to the lone Group 5 entry, this should be avoided by clear instructions and direction as to who is which group, what each group means and if or whether another group in the same class is doing the same lab procedure for the small group data collection. With these minor adjustments, the lab may have reached expected percentages. Part B: Concluding: The illustrations show that when the slide slightly filled with water is doused with saltwater, the water diffuses out of the onion cell into salt water to give more concentration of water in the area outside of the cell. This causes the cell to shrivel a bit. Then water is added once more and plasmolysis occurs, where the cell breaks apart and the slide is filled with onion cells that turn into goo a bit, and look damaged. This is because is plasmolysis, the cell bursts and expands. This is an example of osmosis as the water moves down its concentration gradient, from high concentration (the inside) to low concentration (outside). Evaluating the Procedure: A weakness in this lab was the inability for some students to identify and use the parts of a microscope. Some students had a hard time making the slide also, having a clear view of the onion cell was difficult at first since it has been a couple of years since we have looked at slides and operated a microscope to look at the contents of the slide. Limitations in this lab include that there was a limited amount of time to prepare for and do the lab; there was limited amount of microscopes to be used, and there was exchanging and moving of materials between labs. The little time made people rush to get evaluations and visuals that are not necessarily correct. The limited amount of microscopes caused some people to have to wait, doing nothing, for periods of time to get to a microscope. The limited amount of materials made it more necessary to exchange and move materials that people were in need of. Improving the Investigation: To improve on the weakness, which is lack of knowledge on the students’ part, maybe a class run-through should be held on how and when to use the materials needed and how to interpret them. The limitation of time may be fixed by doing a lab such as this one on a block day instead of during a normal school day. In regards to the limited amount of microscopes, there should be a list of names or some organized method of reaching materials that there are only a few sets or a small number of. This way, the materials are not moved, messed with or mistakenly used wrong since a designated person or a preset schedule will be set for the use of the materials, making the lab run quicker and smoothly as well. This would allow for better understanding and better evaluations and visual drawings on the students’ part, bettering the understanding of the structure of the lab and the end result of the lab. Part C: Concluding: The dialysis bags of each group were filled with the following solutions according the group’s assignment: water, 0.2M sucrose, 0.4M sucrose, 0.6M sucrose, 0.8M sucrose and 1.0M sucrose. Each group would then place the bag into a beaker filled 2/3 with distilled water. The data collected measuring the weight in grams of the dialysis bag, before and after each bag was soaked, shows that the expected values should all have shown an increase in mass. The increase in mass can be attributed to the semi-permeable membrane, which allows certain molecules to pass through it according to their size. Water can move through this membrane, since it is a relatively small molecule, while the sucrose within the bag does not pass through, since it is quite large. This means that water moves down its concentration gradient, in this case from outside the bag (high concentration) to inside the bag (low concentration), to even out its concentration on both sides of the membrane. The bag, though, already has sucrose, which contributes to the dialysis bag’s initial mass. When water diffuses into the bag, mass is added to the bag. This means that the more sucrose is within the bag, the higher its initial mass is, and then the addition of water after osmosis has occurred, coming from the water the bag was soaked in, adds to the mass of the bag, which is what its final mass will be. This correlates with the data collected because the higher in concentration of sucrose the solution within the bag is, the more positive its expected percent change in mass becomes because the added water molecules within the bag after osmosis increase its mass. Evaluating Procedure: A weakness in this lab was that we did not know what other substances were at work and whether they attributed greatly to the increase in mass that was observed in each group but one. Limitations are that the same material was used for all biology classes, such as beakers and solutions. The beakers used during 6th period, which is towards the end of the school day, may have been contaminated by substances that should not have been there, or could have been mistaken for a beaker of sucrose solution with a different concentration, messing up the data. Solutions were also the same for all classes. Certain solutions may have been mistaken for others and used improperly, which would skew the data. Another possibility is that pipettes from Part A of this lab were dipped into one solution, then another, which would contaminate the second solution that was dipped into. This would also skew the data for the group using these certain solutions, and ultimately 6th period and the class average in the percent change in mass of the dialysis bag. Improving the Investigation: A way to absolve the weakness of unknown substances at work would be to check and see for indications of other substances, knowing which indications meant which substance would be present, and also its affect on the increase, decrease or stability of the mass of the dialysis bag. An improvement in regards to possible contamination of beakers and solutions would be to allow each group a specific set of materials that would correspond with the specific solutions, and set them up in different sections of the classroom. This way, no one may get confused as to which solution was of which concentration or which empty beaker contained which solution. Also, emptied or reused materials, such as an empty beaker, pipettes or flasks should be rinsed and washed thoroughly before use so that any chance of contamination is minimized.