AP Biology Diffusion and Osmosis Lab Name ____________
advertisement

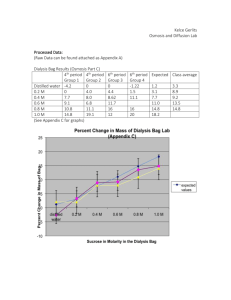
AP Biology Diffusion and Osmosis Lab Name _____________________ Date__________ Names of group members: ________________________________________________________ What causes my plants to wilt if I forget to water them? A laboratory assistant prepared solutions of 1.0 M, 0.8 M, 0.6 M, 0.4 M, 0.2 M sucrose, but forgot to label them. After realizing the error, the assistant randomly labeled the flasks containing these four unknown solutions as flask A, flask B, flask C, flask D, and Flask E. Design an experiment, based on the principles of diffusion and osmosis that the assistant could use to determine which of the flasks contains each of the four unknown solutions. Part 1: Modeling Diffusion And Osmosis Complete this part in pairs. Materials Distilled or tap water 1 M sucrose 1 M NaCl 1 M glucose Elodea—6 leaves total 2 Microscopes Step 1: Start by looking at Elodea under the light microscope. Draw a sketch of 3-5 cells from your leaf at 100X or 400X magnification in your lab notebook. Step 2: Treat each cell with one of the following solutions: 1M sucrose, 1MNaCl, and 1M glucose, and then observe. Step 3: Add Distilled Water to each of your cells and note any changes that occur. At each step, be sure to answer the following questions by labeling your sketch and making descriptive observations in your lab notebook. Where is the cell membrane in relation to the cell wall? Can you see the two structures easily? Why or why not? What parts of the cell that you might see controls the water concentration inside the cell? What changes do you expect to see when the cells are exposed to the each of the solutions? How will you know if a particular treatment is increasing turgor pressure? If it is reducing turgor pressure? How could you determine which concentration of each solution is isotonic to the cells? How do your results compare to your expected results? AP Biology Diffusion and Osmosis Lab Name _____________________ Date__________ Part 2: Design an Experiment to: 1) Determine the concentration of unknown solutions of sucrose 2) Calculate the water potential of potato cells. Materials: Potato Cork borer Balances Rulers Cups 1M,.8M, .6M,.4M,.2M, 0.0M sucrose (Your beakers will be color coded) These questions are just to think about to guide your investigation: How would you determine the molar concentration of the potato? How would you calculate the water potential in the cells? What would your results be if the potato were placed in a dry area for several days before your experiment? When potatoes are in the ground, do they swell up with water when it rains? If not, how do you explain that, and if so, what would be the advantage or disadvantage? Methods: Create a procedure that will help you determine the concentration of the unknown solution of sucrose and calculate the water potential of the potato cells. Write this procedure in your lab notebook. HINT: you will need to let your potatoes sit in solution overnight / until next class. Results: Record all appropriate data in your notebook in a manner that is understandable for anyone who might look at your book. Reporting your Findings: Formally share your results to turn into Mrs. Graham Data Analysis: 1. Calculate percent change in mass of your potato cores. 2. Organize your masses, percent changes, and determined molarities into a data table. 3. Graph the percent change in mass of your potato cores vs. molarity of the solutions. 4. Use your graph to help calculate water potential of the potato. 5. Write a description summarizing your data. Be prepared to answer questions about your lab when you turn in your data. You will be allowed to use all of your lab materials, so remember to bring your lab notebook to class. Adapted from the College Board AP Biology Student Lab Manual 2001 edition and 2012 edition. Further modified by Parul Matani Pre-lab questions: Author AP workshop. Unknown author.