Atoms - Sophia
advertisement

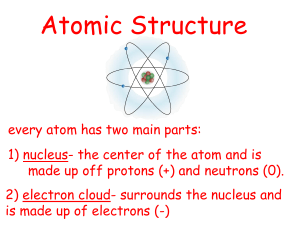
Atoms All elements are made up of atoms. Atoms are the basic particles of all matter Atoms • An atom of Oxygen for example, is the smallest amount of oxygen you can possibly have • Atoms of elements contain protons, neutrons and electrons Charges and Location • Protons= positive charge/inside nucleus • Neutrons= neutral (no) charge/inside nucleus • Electrons= negative charge/outside nucleus in an electron cloud An Atom of Oxygen Ions • An ion is an atom that is either missing electrons or has some extra electrons • Example: When lithium ionically bonds with bromine to form lithium bromide it gives up its one valence electron (outermost electron) to bromine leaving it with 2 electrons rather than 3. Bromine then has 36 electrons rather than 35. Electrolytes • Electrolytes form ions in water and can conduct electricity – Example: Carbonic acid breaks down into ions when dissolved in water H2CO3 H+ + HCO3-