How does water move in the body?
advertisement

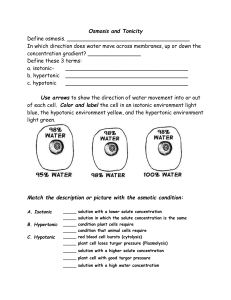
How does water move in the body? • The cell membrane is semi-permeable • Water can move freely • Water is in equilibrium between cells and extracellular fluids (osmotic equilibrium) • Ions and solutes are disequilibrium • Osmosis water moves along its concentration gradient across a semipermeable membrane Distribution of solutes in the body fluid compartments plasma Interstitial fluid Intracellular fluid Ions and solutes are in disequilibrium Ions and solutes are in disequilibrium • Water can cross the cell membrane Na+ Na+ K+ K+ proteins Osmosis • water moves along its concentration gradient across a semi-permeable membrane • Water moves to dilute a solute Osmosis Osmotic pressure is pressure exerted to counter the movement of water to dilute something Osmolarity • Describes the number of particles in solution • Know this and the direction of water movement can be predicted • • • • # of particles in 1 liter of solution Is expressed as osmoles/L, or OsM If very dilute: milliosmoles/L, or mOsM Human body, approx 300 mOsM Osmolarity: number of particles in 1L • 1 M glucose = 1 OsM glucose • 1M NaCl = 2 OsM NaCl, because NaCl disassociates to 2 ions in solution. Na+ Cl- Compare the osmolarity of 2 solutions: • Solution A • Solution B • 1 OsM glucose • 2 OsM glucose • A is hyposmotic to B • B is hyperosmotic to A • (A has fewer particles than B) • (B has more particles than A) Compare the osmolarity of 2 solutions: • Solution B • Solution C • 2 OsM glucose • 1 OsM NaCl • B is hyperosmotic to C • C is hypotonic to B • (B has more particles/L than A) • (C has fewer particles/L than B) Compare the osmolarity of 2 solutions: • Solution A • Solution C • 1 OsM glucose • 1 OsM NaCl • A is isosmotic to C • C is isosmotic to A Osmosis, the diffusion of water across the cell membrane, has consequences on cells • After water leaves a cell, the volume changes (it can shrink) Tonicity • Describes how the cell volume will change in a solution P is penetrating solute N is nonpenetrating solute Water moved into the cell to dilute the solutes. • Cell gains volume in a hypotonic solution • Cell looses volume in a hypertonic solution • Cell keeps the same volume in an isotonic solution. Tonicity indicates how the cell volume will change in a solution • In a hypotonic solution, the cell has a higher concentration of a nonpenetrating solute than the solution, water moves in. • In a hypertonic solution, the cell has a lower concentration of nonpenetrating solute than the solution, water leaves the cell During intavenous injection: • • • • 0.9% (normal) saline isotonic D5--.9% saline (5% dextrose) isotonic D5W hypotonic 0.45% saline hypotonic • Vs dehydration hypotonic • Vs blood loss isotonic