PPTX
advertisement

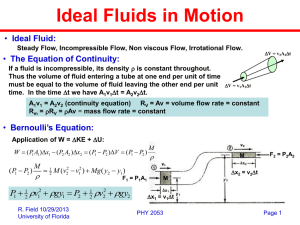
Clicker Question Room Frequency BA Cube A has edge length L and mass M. Cube B has edge length 2L and mass 4M. Which has greater density? A) A has larger density B) B has larger density C) A and B have the same density. Cube A has larger density. ρA = M/L3 , ρB = (4M)/(2L)3 = (1/2)M/L3 so object A has twice the density of object B. 1 Announcements • CAPA assignment #12 is due on Friday at 10 pm. • Next week in Section: Assignment #6 • Finish reading Chapter 10 on Fluids • Midterm 3 scores are uploaded on CU Learn. Solutions are posted on CULearn. Rough grade scale is Average = 62.2 out of 100 Stan. Dev. = 17.3 A range (80-100) B range (60-80) C range (45-60) D range (30-45) F range (0-30) 2 Fluid Density We imagine that the volume V is completely filled with some “continuous” substance. We measure the mass m and the volume V then calculate the mass density ρmass as m/V. Gases: Compressible (expand or compress) Liquids: Often nearly incompressible We’ll typically assume, for a given substance, that the mass density is the same everywhere in side the fluid! This is a good assumption for statics. SI Units are kg/m3 =1000 g/cm3 3 Specific Gravity Another common and useful measure of density is the specific gravity (SG). X Specific Gravity (SG) of substance X Water SGWater = 1 SGIron = 7.9 SGPb = 11.9 SGIce = 0.92 SGAlcohol= 0.79 No “visible” units because it is a ratio! 4 Back to square 2: Fluid “Forces” When we try to apply Newton’s Laws to Fluids what do we use for the force? Again we consider a “small” imaginary volume or box inside the body of fluid. To find the force on the imaginary volume V, we use the concept of pressure. We find the force F on each side of the volume V and divide the magnitude of that force by the area A of that side. The pressure P is then F/A r Fon side of V Pat side of V Aof side V 5 Units of Pressure Pat side of V r Fon side of V Aof side V SI Units of Pressure: N/m2 = 1 Pascal (Pa) English Units of Pressure: lb/in2 = 1 pound per square inch (psi) How do these unit compare in size? 6 Room Frequency BA Clicker Question Approximately(!) what is 1 psi in the SI unit Pa? (1 lb = 4.45 N) A) B) C) D) E) 5 200 7000 4 x 104 1 x 105 Both force and area units have to be converted! 2 lb 5 N 40 in N Guess estimate 1 2 g 8000 2 in lb 1 m m 2 lb 4.45 N 39.4 in N Accurate Calculation 1 2 g 6910 2 1m in lb m 7 Atomic View of Fluid Pressure from Air Air consists mostly of oxygen and nitrogen molecules. At room temperature, the molecules have thermal energy and are moving around rapidly (speed ≈ 400 m/s), colliding with each other and with every exposed surface. The pounding of the air molecules on a surface, like the pitter-pat of rain on the roof, adds up to a large force per area: Patm = 14.7 psi. In a later chapter we’ll calculate the force on the walls directly from these atomic collisions! 8 Too Many Units of Pressure! The most common pressure in everyday life is that coming from the atmosphere (at sea level) ! 1 atmosphere = 1 atm = 14.7 psi = 1.013 x 105 Pa = 1.013 bar = 760 torr 9 Clicker Question Room Frequency BA The air pressure inside the Space Station is P = 12 psi. There are two square windows in the Space Station: a little one and a big one. The big window is 30 cm on a side. The little window is 15 cm on a side. How does the pressure on the big window compare to the pressure on the little window? A) same pressure on both windows B) 2 times more pressure on the big window C) 4 times more pressure on the big window D) 9 times more pressure on the big window 10 Clicker Question Room Frequency BA The air pressure inside the Space Station is P = 12 psi. There are two square windows in the Space Station: a little one and a big one. The big window is 30 cm on a side. The little window is 15 cm on a side. How does the total force on the big window compare to the total force on the little window? A) same force on both windows B) 2 times more force on the big window C) 4 times more force on the big window D) 9 times more force on the big window F = P*A 11 Direction of Fluid Forces Newton’s law deals with forces which are vectors! What is the direction of the force from fluid pressure? For static fluids (no flow) the force is perpendicular to the surface of the side. In the figure you see arrows for the force on each side of an imaginary cube of volume V. Critical point: Pressure does not have any direction; the direction of the force from pressure depends on the orientation of the surface the pressure acts on. 12 Is 15 psi a big pressure? Yes!!! We are normally unaware of this because forces on us are balanced. Here are two video examples and a demo! 13 Applying Outside Pressure to a Fluid Pascal’s Principle: If an external pressure is applied to a confined fluid, the pressure at every point within the fluid increases by that amount. POUT PIN FOUT FIN AOUT AIN FOUT AOUT FIN A IN Example: Hydraulic lift 14 Pressure in Fluids on a Planet I For our first application of Newton’s 2nd Law on Fluids, let’s consider the result of gravity on the pressure in a fluid. Consider an imaginary cube with each side of area A, the top at the surface and the bottom at depth h. Patm Each of the six faces contributes to the net force! Four side faces cancel each other; what about top and bottom faces? Fnet = Ftop + Fbottom = 0 Fnet = -PatmA + PhA - Mwaterg = 0 Ph 15 Pressure in Fluids on a Planet II Fnet = -PatmA + PhA - Mwaterg = 0 Ph A Patm A M Water g Patm M Water g Ph Patm A Ph Patm Water (Ah)g A Patm Water hg Ph Pressure increases linearly with depth, proportional to g and ρ! 16 More on Gravity and Pascal’s Principle Ph Patm Water hg Pressure only depends on the depth; at a given depth the pressure is the same. Often people leave out the Patm, but if you want the total, absolute pressure, you must include it. Gauge Pressure is the amount of pressure after subtracting Patm How do you know what kind of gauge you are reading? 17 Example. At the surface of a swimming pool the pressure on a swimmer is due to air: Patm = 1 atm. At what depth in the pool will the pressure be 2 atm? Ph Patm gh Ph Patm h g 1 atm ~ 105 Pa 2x10 1x10 Pa h 10 m 3 2 (1000kg/m )(10 m/s 5 5 18 Clicker Question Room Frequency BA As shown, two containers are connected by a hose and are filled with water. Which picture correctly depicts the water levels? Different depths would give different pressures! The net force on the fluid at the connection tube would not be zero and there would be flow! P gh 19