Powerpoint slides
advertisement

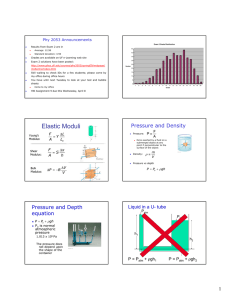
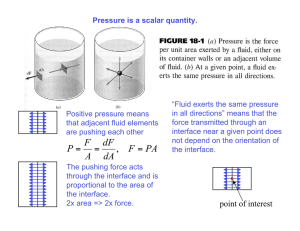
Physics 101: Lecture 23 Fluids: Gases and Liquids Today’s lecture will cover Textbook Sections 11.1-11.4 Density Pressure Physics 101: Lecture 23, Pg 1 Density Density = Mass/Volume = M/V SI unit: [kg/m3] Densities of some common things (in kg/m3): Water (at 4 degrees Celsius) 1000 ice 917 (floats on water) blood 1060 (sinks in water) lead 11,300 Copper 8890 Mercury 13,600 Aluminum 2700 Wood (yellow pine) 550 air 1.29 Helium 0.179 For comparisons of densities we use: Specific gravity = density of substance/density of water Physics 101: Lecture 23, Pg 2 Pressure The magnitude of a force acting perpendicular to a surface per unit area is called pressure: P=F/A SI Unit: [N/m2 ] N/m2 is also called Pascal [Pa]. 105 Pa = 1 bar of pressure Because of pressure any gas or liquid applies a force perpendicular to the surface with which it comes in contact. The air above the surface of the earth creates a pressure at sea level of (=atmospheric pressure) 1.013 x 105 Pa = 1 atmosphere Physics 101: Lecture 23, Pg 3 Pressure and Depth in a Static Fluid In a static fluid when knowing the pressure at a certain depth, P1 at d1, the pressure at a larger depth, P2 at d2, can be calculated from the increase in pressure due to the weight of the fluid that is located between d1 and d2: P2 A = P1 A + m g = P1 A + V g P2 = P1 + h g (h=d2-d1) Here we assumed that the fluid is not compressible, i.e. its density is the same at any depth. Physics 101: Lecture 23, Pg 4 Pressure and Depth Barometer: a way to measure atmospheric pressure P2 = P1 + g h Patm = g h Measure h, determine Patm or P1=0 Example: Mercury = 13,600 kg/m3 Patm = 1.05 x 105 Pa P2=Patm h h = 0.757 m = 757 mm = 29.80” (for 1 atm) Open tube manometer: P1 = Patm P2-Patm = ρ g h : gauge pressure P2 : absolute pressure Physics 101: Lecture 23, Pg 5 Concept Question Suppose you have a barometer with mercury and a barometer with water. How does the height hwater compare with the height hmercury? 1. hwater is much larger than hmercury 2. hwater is a little larger than hmercury 3. hwater is a little smaller than hmercury 4. hwater is much smaller than hmercury CORRECT water is much less dense than mercury, so the same amount of pressure will move the water farther up the column. P1=0 P2=Patm Pa gh h h Pa g mercury 13.6 water so, h water 13.6 h mercury Physics 101: Lecture 23, Pg 6 Concept Question P=0 Pa Pa gh h Pa g h Evacuate the straw by sucking. How high will the water rise? no more than h = Pa/g = 10.3m = 33.8 feet no matter how hard you suck! Physics 101: Lecture 23, Pg 7 Concept Question Is it possible to stand on the roof of a five story (50 foot) tall house and drink, using a straw, from a glass on the ground? 1. No CORRECT 2. Yes Even if a person could completely remove all of the air from the straw, the height to which the outside air pressure moves the water up the straw would not be high enough for the person to drink the water. Physics 101: Lecture 23, Pg 8 Summary • Density: = M/V • Pressure: P = F/A P2 = P1 + g h Physics 101: Lecture 23, Pg 9