Matter and its Properties For the element sulfur, create a table of the
Matter and its Properties
1.
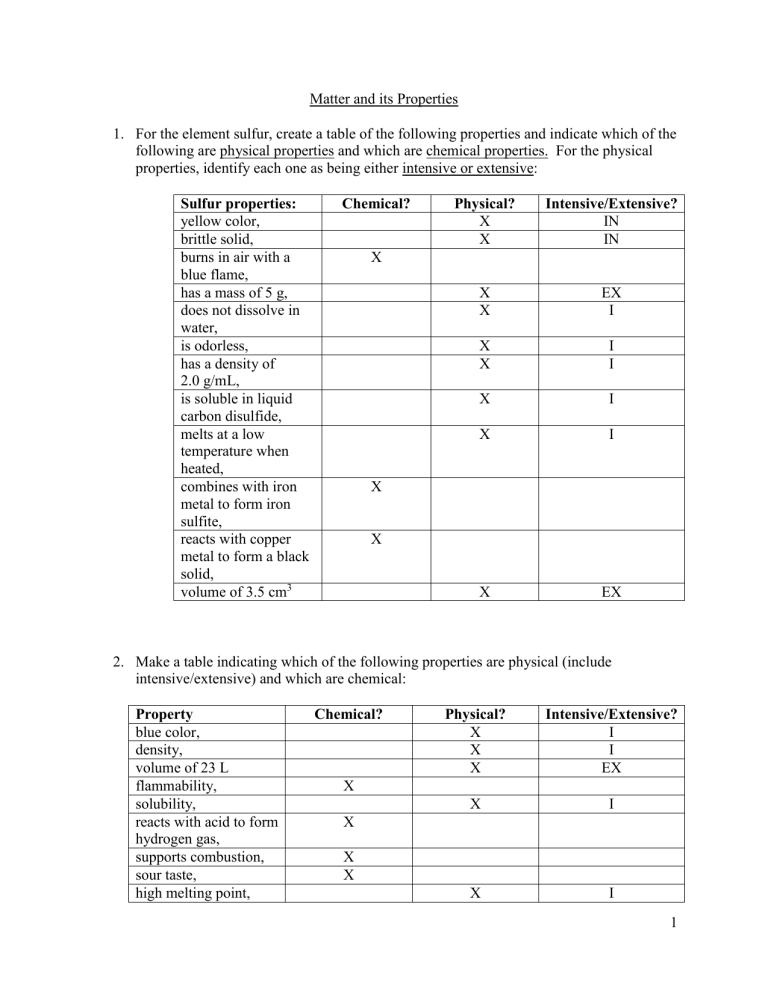
For the element sulfur, create a table of the following properties and indicate which of the following are physical properties and which are chemical properties. For the physical properties, identify each one as being either intensive or extensive:
Sulfur properties: yellow color, brittle solid, burns in air with a blue flame, has a mass of 5 g, does not dissolve in water, is odorless, has a density of
2.0 g/mL,
Chemical?
X
Physical? Intensive/Extensive?
X
X
X
X
X
X
IN
IN
EX
I
I
I
X I is soluble in liquid carbon disulfide, melts at a low temperature when heated,
X I combines with iron metal to form iron sulfite, reacts with copper metal to form a black solid, volume of 3.5 cm 3
X
X
X EX
2.
Make a table indicating which of the following properties are physical (include intensive/extensive) and which are chemical:
Property blue color, density, volume of 23 L flammability, solubility, reacts with acid to form hydrogen gas,
Chemical?
X
X
Physical?
X
X
X
X
Intensive/Extensive?
I
I
EX
I supports combustion, sour taste, high melting point,
X
X
X I
1
reacts with water to form a gas, hardness, low boiling point, luster
Odor
Mass of 28 g
X
X
X
X
X
X
I
I
I
I
EX
3.
Circle the following that are physical changes and underline the chemical changes : a.
kicking a football, milk spoiling, rusting iron, cutting paper, making a cloth from cotton, tarnishing of silver metal, melting butter, dissolving sodium hydroxide pellets in water, reacting hydrochloric acid with potassium hydroxide, slicing a piece of sodium, heating water and changing it to steam, decomposing potassium chlorate into potassium chloride and oxygen gas, evaporation, ice melting, sugar dissolving in water, wood rotting, pancakes cooking on a griddle, grass growing, inflating a tire, food digesting, water being absorbed by a paper towel, dynamite exploding, breaking the windshield of a car, developing photographic film, combustion of coal in a furnace, excavation of earth, electrolysis of water, expansion of steel metal in a bridge in hot weather, condensation of water vapor on a mirror, mothballs disappearing over time, erosion of a riverbed, leaves changing color in the fall, fireworks exploding, and gallium melting in your hand.
4.
State the Law of Conservation of Mass:
In any physical or chemical change matter in neither created or destroyed; it is conserved
5.
What are the two types of matter?
Pure substances and mixtures.
6.
Name the two types of mixtures and identify which type has its components uniformly distributed throughout and which has components that are not uniformly distributed
2
Homogenous mixtures are uniform (all parts of mixture are exactly the same)
Hetereogenous mixtures are not uniform (substances remain distinct even when mixed)
7.
Which type of matter is made up of only one type of atom or molecule?
Pure substances
8.
Name the two types of pure substances. a.
Which type of matter consists of only one kind of atom?
Elements b.
Which type of matter consists of two or more different atoms chemically reacted together?
Compounds
9.
Can all pure substances be separated? Explain. If yes, list ways to separate them.
Not by physical methods (only through chemical reactions)
10.
Can all mixtures be separated? Explain. If yes, list ways to separate them.
Yes, all mixtures can be separated since the substances are not chemically bound.
Several ways to separate them include: distillation, filtering, evaporation, decanting, centrifugation, crystallization, chromotagraphy and even simply picking out different pieces!
11.
Make a table identifying each of the substances below as pure substances or mixtures, then classifying them further as either elements, compounds, homogeneous mixtures, or heterogeneous mixtures: a.
sodium chloride (table salt), an apple, plutonium, carbon monoxide, acid rain, calcium, smog, paint, ammonia, calcium sulfide, gold, chicken soup, milk, blood, air, methanol, tantalum, argon, iced tea, silver, orange juice, sodium bicarbonate
(baking soda), ammonium chloride, spaghetti sauce, methane, antimony, salad, the atmosphere, carbon dioxide, potassium, chili, and sulfuric acid.
Substance Pure
Substance
X
Element Compound Mixture Homogeneous
Mixture
X
Heterogeneous
Mixture sodium chloride
Apple
Plutonium carbon
X
X
X
X
X X
3
X
X
X
X
X
X
X
X
X
X
X
X
X
X iced tea
Silver orange juice sodium bicarbonate
Ammonium chloride spaghetti sauce
Methane
Antimony atmosphere carbon dioxide
Chili sulfuric acid monoxide acid rain
Calcium
Smog
Paint
Ammonia calcium sulfide
Gold chicken soup
Milk
Blood
Air
Methanol
Tantalum
Argon
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
Possibly
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
4
Scientific Method
Word Bank:
Chart
Communicate
Conclusion
Constant Factors
Control
Dependent
Environment
Experiment
Graphs
Hypothesis
Independent
Materials
Observations
Procedures
Purpose
Qualitative
Quantitative
Rigorous
Results
Scientific Law
Theory
12.
Match the words above with the following six definitions:
Word Definition
Independent
Theory
A variable that the scientist changes or controls in order to do the experiment.
A generally accepted explanation of a natural phenomenon that has been tested many times.
Hypothesis
A tentative, educated guess about how something works.
Conclusion
Graphs
A judgment based on the information obtained on whether your experiment did or did not support your hypothesis.
A type of data report that uses drawings to help determine and display relationships and trends.
Information gathered using human senses that describes color, odor, shape or other physical characteristic. Qualitative data
13.
List in order the five major steps a scientist follows under the Scientific Method:
1.
Make Observations .
2.
Develop Hypothesis .
3.
Perform Experiment (including analysis) .
4.
Draw Conclusions .
5.
Communicate results .
5
14.
A student wants to determine which of three chemicals makes the best ‘cold pack’ for treating physical injuries. Her schematic of the experiment is shown below.
Cup:
Water (mL) 50 50 50
Chemical, Identity:
Chemical, Amount (g)
Temp. Initial ( o
C)
Temp. Final ( o C)
CaCl
2
2
22
29
.
.
.
0
4
0
NH
2
23
18
.
.
.
4
Cl
0
5
2
NaCl
2
22
23
.
.
.
0
6
7
What was the INDEPENDENT VARIABLE(S)? Why?
The identity of the chemical. This was what the scientist changed between cups A, B and
C.
What was the DEPENDENT VARIABLE(S)? Why?
The change in temperature. This variable changed based on the changing of the chemical.
What was the control(s)?
The type and size of the cup and the amount of water in it.
What was her likely hypothesis?
If the type of chemical dissolved in water is changed, the amount of cooling (temperature reduction) will vary because of different interactions of each chemical with the water.
How could the student have improved the experiment?
She could have done multiple trials (other improvements are also possible).
6
Scientific Notation, Measurements and SI Units
15.
Express the following numbers using scientific notation: a.
1,000,000 = 1 x 10 6 b.
c.
230 = 2.3 x 10
1 d.
e.
24, 212,000 = 2.4212 x 10
7 f.
0.0095 = 9.5 x 10
0.8 = 8 x 10
-1
-3
0.000665 = 6.65 x 10
-4 g.
21.9 = 2.19 x 10 1 h.
0.00332 = 3.32 x 10
-3 i.
j.
0.12011 = 1.2011 x 10
-1 k.
3210 = 3.21 x 10
45,700 = 4.57 x 10 l.
0.00001= 1 x 10
-5
3
5
16.
Express the following numbers in expanded (ordinary) form: a.
3.825x10
3 = 3,825 b.
6.3x10
4 c.
2.3x10
-2
= 63,000
= 0.023 d.
4.44x10
-6 = 0.00000444 e.
7.121x10
5
= 712,100 f.
1.8x10
2
= 180
17.
Write each of the following as an “ordinary” decimal number: a.
6.235x10
-2
= 0.6235 b.
7.229x10
3
= 7,229 c.
5.001x10
-6 d.
8.621x10
4
= 0.000005001
= 86,210 e.
4.83x10
2 g.
6.1x10
0
= 483 f.
7.221x10
-4
= 0.0007221
= 6.1 h.
9.11x10
-8 = 0.0000000911
7
18.
Calculate answers for the following and write them in standard scientific notation: a.
1/0.00032 = 3.125 x 10
3 b.
1000/0.002 = 5 x 10
5 c.
1250/1000 = 1.25 x 10
0 d.
1/55,000 = 1.8 x 10 -5 e.
(15,000 x 850 x 0.0035)/0.0022 = 2.0 x 10
7 f.
43.2/0.000432 = 1.00 x 10
5 g.
5.58/(10,000 x 0.000001) = 5.58 x 10
2
19.
Solve the following and express your answer in scientific notation: a.
6.6x10
-8
/3.3x10
-4
= 2.0 x 10
-4 b.
7.4x10
10
/3.7x10
3
= 2.0 x 10
7 c.
2.67x10
-3
– 9.5x10
-4
= 1.72 x10
-3 d.
1.56x10
-7 + 2.43x10
-8 = 1.80 x 10 -7 e.
2.5x10
-6
x 3.0x10
-7
= 7.5 x 10
-13 f.
1.2x10
-9
x 1.2x10
7
= 1.4 x 10
-2 g.
2.3x10
4
+ 2.0x10
-3
= 2.3 x10
4
8
20.
Give the SI unit and abbreviation for each of the following quantities: a.
Temperature = Kelvin (K) e.
volume = liter (L) b.
mass = gram (g) c.
time = second (s) f.
amount of a substance = mole
(mol), used for counting d.
length = meter (m) atoms and molecules.
21.
Write the meaning (as a power of 10) for each of the following metric prefixes: a.
kilo = 10
3 d.
deci = 10
-1 b.
centi = 10
-2 c.
milli = 10
-3 e.
nano = 10
-9 f.
micro = 10
-6
22.
Give the metric prefix that corresponds to each of the following meanings: a.
one million = mega e.
10 -3 = milli b.
d.
one tenth = deci c.
10
-2
= centi one thousand = kilo f.
10 3 = kilo g.
10
-9
= nano h.
10
-6
= micro
23.
Use the correct prefix and unit abbreviation to write out: a.
The mass of 6.6 thousand grams = 6.6 kg b.
The length of 0.00245 meters = 2.45 mm c.
The time of 0.0000000067 seconds = 6.7 ns
24.
Convert 45 degrees Celsius to Kelvin and Fahrenheit.
113 o F, 318 K
25.
Convert 76 degrees Fahrenheit to Celsius and Kelvin.
24 o C, 297 K
26.
Convert 500 Kelvin to Celsius and Fahrenheit.
227 o C, 441 o F
9
Significant Figures
27.
Determine the number of significant figures in each of the following measurements: a.
0.02 = 1 g.
6051.00 = 6 m.
0.90100 = 5 b.
c.
0.020 = 2
501 = 3 h.
i.
0.0005 = 1
10001 = 5 n.
90100 = 3 o.
4.7x10
-8 = 2 d.
e.
f.
501.0 = 4
5000 = 1
5000. = 4 j.
8040 = 3 k.
0.030 = 2 l.
2.000x10
2 = 4 p.
10800 = 3 q.
3.01x10
21 = 3 r.
0.000410 = 3
28.
Solve the following problems, giving the final answer with the correct number of significant figures and the correct units: a.
2.674 m / 2.0 m = 1.3 m b.
5.25 L x 1.3 L = 6.8 L c.
9.0 cm + 7.66 cm + 5.44 cm = 22.1 cm d.
10.07 g – 3.1 g = 7.0 g
29.
Identify the number of significant figures in each of the following: a.
0.025 g = 2 d.
0.0404 nm = 3 g.
5.50x10
3
yd = 3 b.
c.
40.0 m = 3
0.0081 sec = 2 e.
f.
12 in = 1 foot = infinite
129.042 Hz = 6 h.
500 miles = 1 i.
273K = 0 o
C = infinite
30.
Solve the following math problems and express your final answers with the correct number of sig figs and the correct units: a.
12.62 + 1.5 + 0.25 g = 14.4 g b.
4.68 x 12.5 g = 5.85 x 10
1
g c.
23.6 x 1.02 / 2.3 g = 1.0 x 10
1 d.
(1.245 + 0.03 + 32) / 54.6 mL = 0.60 g (first part rounds to 33 using sig fig rules for addition, then divide by 54.6 and round to two sig figs based on the 33 figure) e.
45.6 – 3.2 K = 42.4 K f.
3.482 cm + 8.51 cm + 16.324 cm = 2.83 cm g.
48.0032 g – 16.532 g = 31.471 g h.
48.2 cm x 1.6 cm x 2.1212 cmc= 1.6 x 10
2 cm i.
0.0575 mL x (760mm Hg / 745 mm Hg) x (273 K / 256 K) = 6.3 x 10
-2
mL
10
Density
31.
Define density: the amount of matter per unit of volume. Density = Mass/Volume
32.
Which has the highest density: 10 kilograms of iron or 20 kilograms of marshmallows?
Iron (note that the amount of grams of the substance is not what matters here)
33.
A sample has a mass of 50.0 g and a volume of 25.0 ml. What is its density?
D=M/V, thus D = 50.0g / 25.0 mL = 2.00 g/mL
34.
Sulfur has a density of 2.070 g/cm
3
. What is the volume of 10.0 g of sulfur?
4.83 cm 3
35.
How much space is occupied by 2.50 g of aluminum, if its density is 2.700 g/cm 3 ?
0.926 cm 3
36.
Mercury has a density of 13.550 g/cm
3
. What is the mass of 10.0 mL of mercury?
136 g
37.
Gold has a density of 19.300 g/cm
3
. How much matter is contained in 5.00 cm
3
of gold?
96.5 g
38.
A cube has a mass of 15.0 g. It measures 2.31 cm on a side. What is the volume of the cube? What is the density of the cube?
Volume = (2.31cm) 3 = 12.3 cm 3
Density = 15.0g/12.3cm
3 = 1.22 g/cm 3
39.
A volume of 10.0 mL of alcohol is placed in a graduated cylinder. The density of the alcohol is 0.781 g/mL. When a 50.0 g fishweight (a solid ball of metal) is added to the cylinder, the alcohol level goes up to 16.0 mL. a.
What is the volume of alcohol displaced? = 6.0 mL b.
What is the volume of the fishweight? = 6.0 mL c.
What is the density of the fishweight? = 50.0g/6mL = 8 g/mL d.
What is the mass of alcohol displaced? = 6.0mL x 0.781 = 4.7 g e.
If the experiment were repeated with water, what volume of water would be displaced? = 6.0 mL
11
f.
What mass of water would be displaced? = 6.0 g
12
40.
The density of a metal bar is 2.4 g/mL. What is the specific gravity of the bar? specific gravity (compare to water density) = 2.4g/mL / 1.0g/mL = 2.0 (no units)
41.
A 25.0 g fishweight is placed in a graduated cylinder massing 50.00 g. If the water level raises from 15.00 mL to 20.50 mL then a.
What is the volume of the fishweight? = 5.50 mL b.
What is the density of the fishweight? = 25.0g /5.50 mL = 4.55 g/mL c.
What is the mass of water displaced by the fishweight? = 5.50g d.
If the fishweight were placed in alcohol (D = 0.910 g/mL), what volume and mass of alcohol would be displaced?
= Volume = 5.50 mL, mass = 5.00g
42.
A student performs a density lab. She is trying to determine the density of an unknown liquid. She takes an empty soda can and masses it on the balance; its mass empty is
50.00 g. Filled completely with water, it masses 110.00 g. She then empties it, dries it, and refills it with the unknown liquid. The soda can now masses 125.00 g. a.
What is the known density of water? = 1.000g/mL b.
What is the volume of the soda can? = 110.00g x (mL/1.000g) = 110.0 mL c.
What is the density of the unknown liquid? = 125.00g/110.0mL = 1.136 g/mL
43.
A metal plate (slab) has dimensions 6.20 cm wide x 10.00 cm long x 6.50 mm high. It has a mass of 140.00 g. a.
What is the surface area of the slab (in cm
2
)? = 62.0 cm
2 b.
What is the volume of the slab (in cm
3
)? = 403 cm
3 c.
What is the density of the metal? = 140.00g/403cm = 0.347 g/cm
3 d.
What is the specific gravity? = 0.347
13
Accuracy and Precision (Error Analysis)
44.
Define accuracy: how close your results are to the accepted value
45.
Define precision: how close your results are to each other (their average)
46.
Determine the percent error if a student does a density lab and calculates the density of mercury to be 13.450 g/mL and the actual density of mercury is 13.550 g/mL.
% error = [13.550 – 13.450 / 13.550] x 100 = 0.73801%
47.
A lead ball is massed four times. Determine the precision error of these measurements if the five trials are as follows: a.
5.43 g b.
5.41 g c.
5.51 g d.
5.39 g
Trial
Results
5.43
5.41
5.51
5.39
Average: 5.435
Absolute
Difference
0.005
0.025
0.075
0.045
Precision
Error %
0.09
0.46
1.38
0.83
0.69
The average of all the errors on each trial is 0.69%, thus this is the precision error for the overall experiment.
48.
Accuracy and precision can be determined for a series of data. Review Table 1 below and then provide answers to the following questions.
Table 1: Data collected by three different students for an object with an accepted value
(AV) for density of 1.59 g/mL.
Trial Student A Student B Student C
1 1.54 g/mL 1.40 g/mL 1.70 g/mL
2 1.60 g/mL 1.68 g/mL 1.69 g/mL
3 1.57 g/mL 1.45 g/mL 1.71 g/mL
14
A.
Given the experimental data in Table 1, use your “eyeball” judgment to identify and explain which student’s data is:
Criteria most accurate
Student Explain your judgment least accurate most precise least precise
B.
Use the following calculations of average, percent error and precision error for each student to check your initial judgment of the results in Table 1:
Average:
Student A Student B Student C
1.57 1.51 1.70
Percent error: [|(AV – Experimental Value)| / AV] x 100
-Use average of each student’s trial results as the EV and 1.59 g/mL as the AV
Student A: [(1.59 – 1.57) / 1.59] x 100 = 1.26% (1% using sig fig)
Student B: [(1.59 – 1.51) / 1.59] x 100 = 5.03% (5% using sig fig)
Student C: [(1.59 – 1.70) / 1.59] x 100 = 6.92% (6.9% using sig fig)
Precision error: [|(Average - Experimental Value)| / Average] x 100
- Determine precision error for each trial, then calculate average precision error for the student.
Student A Precision Error
Trial 1
Trial 2
Trial 3
Trial Experimental
Value (EV)
1.54
1.60
1.57
Absolute Value Error
|(Average – EV)|
0.03
0.03
0.00
1.9%
1.9%
0.0%
Average
Precision Error: 1.3%
15
Student B
Trial 1
Trial 2
Trial 3
Trial Experimental
Value (EV)
1.40
1.68
1.45
Absolute Value Error
|(Average – EV)|
0.11
0.17
0.06
Precision Error
7.3%
11.3%
4.0%
Average
Precision Error:
Student C
Trial 1
Trial 2
Trial 3
Average
Precision Error:
Trial Experimental
Value (EV)
1.70
1.69
1.71
Absolute Value Error
|(Average – EV)|
0.00
0.01
0.01
7.5%
Precision Error
0.00%
0.59%
0.59%
0.39%
C.
Did this error analysis cause you to change your opinion of the results? Why?
Student A was the most accurate (1.26 percent error) and Student C was the most precise
(0.39% precision error)
16