Lewis Dot Structures and an Introduction to Molecular Geometry
advertisement

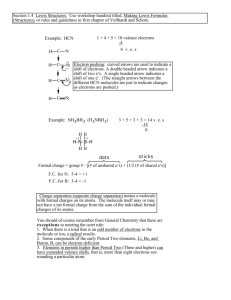
Lewis Dot Structures and Molecular Geometry Chapter 8 in online text Chapter 15 in desk text Why STUDY? Warm-Up What is the electron configuration of carbon? 1s22s22p2 How many valence electrons does Carbon have? 4 To determine the Lewis dot structure for Carbon we look at the number of valence electrons carbon has. Carbon has four valence electrons and we represent electrons as DOTS. C Lewis Dot Structures for the first 10 elements on the periodic table. Chemists use Lewis Dot Structures to determine how elements are covalently bonded to each other in a molecule. • Covalently Bonded means to share electrons between elements. Sharing of electrons occurs between a nonmetal and another nonmetal. • Single Bond- 2 electrons are shared • Double Bond- 4 electrons are shared • Triple Bond- 6 electrons are shared Draw the Lewis Dot structure for BeF2. 1. Count the number of valence electrons you have to work with. Be F Total : valence electrons 2. Put the least electronegative element in the center (remember the trend of E.N) **One exception: we never put Hydrogen in the center, because it can only make one bond. 3. Use pairs of electrons to form bonds between all atoms. (2 electrons make one bond) 4. Use the remaining electrons to complete the octet rule for each element. (Exception: Be disobeys the octet rule it only has two valence electrons) 5. If you run out of electrons, you must introduce multiple bonds to maintain the octet rule. (BeF2 does not have multiple bonds-we will do more examples of this) Let’s try to draw a Lewis Dot Structure of CO2 using the Rules 1. Count the number of valence electrons you have to work with. C O Total : valence electrons 2. Put the least electronegative element in the center (remember the trend of E.N!!!?) O C O 3. Use pairs of electrons to form bonds between all atoms. (2 electrons make one bond) 4. Use the remaining electrons to complete the octet rule for each element. 5. If you run out of electrons, you must introduce multiple bonds to maintain the octet rule. Let’s try NH3 Try these with a partner…. H CH4 H C H H H2O H HCN C N Valence Shell Electron Pair Repulsion VSEPR Electron pairs surrounding an atom want to be as far apart from each other as possible. This gives rise to different molecular shapes. The simplest shape is a LINE Alkali Metals and Halogens make a line because they can only bond once. Groups 1 and 17 make a line Let’s look at another simple molecular shape The electron pairs are the farthest apart they can be. This molecule is LINEAR The center atom bonds twice. Elements in group 2 make linear structures. How is the Be atom able to covalently bound to the fluorines? Hybridization of the orbitals explains this. Two or more of the orbitals of the central atom must mix to form new orbitals of equal energy so bonding can take place. Fill in valence electrons Hybrid mixing 2p s p A linear molecule has sp hybridization 2s Trigonal Planar Shape BH3 Draw Lewis Structure: This arrangement lets all the electron pairs be the furthest apart from each other. Group 13 makes a trigonal planar shape Draw electron diagram for B Hybrid mixing 2p 2s s p p A trigonal planar molecule has a sp2 hybridization Tetrahedral What does the word ‘tetra’ mean? CH4 Methane Structure: This arrangement lets all the electron pairs be the furthest away from each other. Group 14 makes tetrahedral shapes. Draw the electron diagram of Carbon What do you think is the hybridization of tetrahedral molecules?? 2p 2s Trigonal Pyramidal NH3 This arrangement lets all the electron pairs be the furthest apart from each other. With one lone pair. This is similar to the tetrahedral arrangement ,but has one lone pair. Group 16 makes Trigonal Pyramidal Shapes What is the hybridization of NH3( trigonal pyramidal) Fill in valence electrons for Nitrogen 2p 2s Bent shape H2O This arrangement lets all the electron pairs be the furthest apart from each other. With two lone pairs. What is the hybridization of the bent molecule? Do you think it will be similar to tetrahedral? Group 15 makes bent shapes. What is the hybridization of H20? (bent shape) Fill in valence electrons for oxygen 2p 2s VSEPR Theory Summary Group 2 Group 13 Group 14 Group 15 Group 16 Shape Linear #of atoms 2 Hybridization sp Examples CO2 Trigonal Planar 3 sp2 BH3 Tetrahedral 4 sp3 CH4 Trigonal Pyramidal 3 with one lone pair sp3 NH3 Bent 2 with two lone pairs sp3 H2O Polarity of Molecules We can determine if a molecule is polar or nonpolar by the molecule’s shape. In some covalent bonds electrons are not shared equally between the elements. The more electronegative element ‘WINS’ the electrons. The charge difference across the bond as well as the shape of the molecule results in a POLAR Molecule. Polarity of Water Lets take water for example: Polar molecules act as tiny dipoles because they have un even charges. A dipole is created by equal but opposite charges that are separated by a short distance. Direction of dipole is from positive end to more negative end (more electronegative element) Because WATER is a BENT Molecule the polarities of each bond add together to make this molecule highly POLAR What about NH3 Trigonal Pyramidal POLAR OR NONPOLAR? The dipoles of the three bonds are additive combining to form an overall net dipole Because NH3 is trigonal pyramidal shape it is POLAR CH4 CO2 The individual bond polarities extend equally and symmetrically in different directions canceling out . OVERALL the molecules are NONPOLAR. What about CH3Cl? NonPolar or Polar? Try with a partner… Cl H H H POLAR