File
advertisement
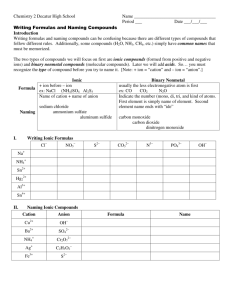
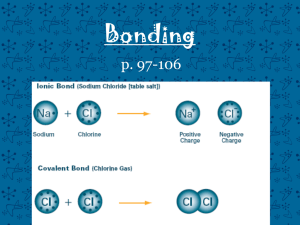
CHEMICAL NOMENCLATURE 1. Binary Ionic Compounds - Type I 2. Binary Ionic Compounds - Type II 3. Ionic Compounds & Polyatomic (Complex) Ions 4. Hydrated Ionic Compounds 5. Binary Covalent Compounds Definitions An IONIC COMPOUND consists of a metal cation bonded to a nonmetal anion. Electrostatic attraction holds them together. A COVALENT COMPOUND consists of two nonmetal atoms sharing valence electrons. A BINARY compound is one that is made of just two elements. Type I Binary Ionic Compounds The metal cations in these compounds have only ONE possible charge. Na+ Zn2+ sodium zinc Al3+ Ca2+ aluminum calcium The charges are memorized or predicted using a periodic table! The cations are bonded to nonmetal anions: O 2oxide N3nitride Ffluoride Br bromide Notice that simple anions are always named with the suffix “ide” In an ionic compound, the charges of the cations and anions must always cancel out. Subscripts are used if more than one atom is needed to cancel the charges: sodium chloride: Na+ and Cl- NaCl lithium oxide: Li+ and O2- Li2O aluminum bromide: Al3+ and Br - AlBr3 zinc nitride: Zn2+ and N3- Zn3N2 potassium iodide: K+ and I- KI silver phosphide: Ag+ and P3- Ag3P Examples: Type I Binary Ionic Compounds Write the formulas: Write the names: •potassium oxide • K3N • zinc chloride • AgI • silver sulfide • ZnBr2 • aluminum nitride • Al2O3 • gallium oxide • Ba3P2 •calcium iodide • LiH Type II Binary Ionic Compounds These are ionic compounds where the metal cation can form TWO different charges. Fe2+ iron (II) Fe3+ iron (III) Ni2+ Ni3+ nickel (II) nickel (III) Co2+ cobalt (II) Co3+ cobalt (III) Cu+ copper (I) Cu2+ copper (II) Au+ gold (I) Au3+ gold (III) Sn2+ tin (II) Sn4+ tin (IV) An older system uses suffixes and Latin names, -ous for the lower charge, -ic for the higher charge: Ferrous & Ferric, Cuprous & Cupric, Stannous & Stannic Examples: Type II Binary Ionic Compounds Write the formulas: Write the names: • iron (II) oxide • Fe2O3 • copper (II) chloride • SnS • tin (IV) sulfide • NiBr2 • cupric nitride •CuS • nickel (III) oxide • Pb3P2 • ferrous iodide • CuBr •cobalt (III) selenide • FeCl3 Polyatomic (Complex) Ions All of the cations and anions so far have been simple ions - single atoms that have lost or gained electrons. A molecule is a particle that forms when two or more atoms bond together. A complex ion is a charged molecule. Complex ions may be cations or anions. examples: nitrate: NO3- sulfate: SO42- hydroxide: OH- Common Polyatomic Ion Compound Naming Examples… a) Ammonium chloride NH4Cl b) Silver sulfate Ag2SO4 c) Aluminum hydroxide Al(OH)3 d) Calcium phosphate Ca3(PO4)2 e) Iron (III) nitrite Fe(NO2)3 f) Copper(II) permanganate Cu(MnO4)2 g) Ammonium dichromate (NH4)2Cr2O7 h) Zinc acetate Zn(CH3COO)2 Things to Notice Most complex ions are anions. Ammonium, NH4+, is the most common complex cation. Several complex ions form a short series of ions. The ions differ only in the number of oxygen atoms: perchorate ClO4- sulfate SO42- chlorate ClO3- sulfite SO32- chlorite ClO2- hypochlorite ClO- nitrate NO3- nitrite NO2- If an ion carries a charge like “-2” or “-3”, a series of related ions can be formed by adding hydrogen cations (H+) while still leaving a net charge: examples: Sulfide: S2- hydrogen sulfide: HS(bisulfide) Sulfate: SO42- hydrogen sulfate: HSO4(bisulfate) Carbonate: CO32- hydrogen carbonate: HCO3- (bicarbonate) Phosphate: PO43- hydrogen phosphate: HPO42- dihydrogen phosphate: H2PO4- More Formulas with Complex Ions a) Sodium bicarbonate NaHCO3 b) Nickel (II) hydrogen sulfide Ni(HS)2 c) Aluminum perchlorate Al(ClO4)3 d) Barium dihydrogen phosphate Ba(H2PO4)2 e) Iron (III) sulfite f) Cuprous bisulfate Fe2(SO2)3 CuHSO4 g) Zinc periodate h) Lithium selenite Zn(IO4)2 Li2SeO3 Hydrated Ionic Compounds A HYDRATE is an salt that has water molecules trapped within its crystals. Every hydrate has a certain number of water molecules associated with each formula unit of the ionic compound. The number of water molecules is indicated by using prefixes. 1 mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca CuSO4 · 5 H2O copper (II) sulfate pentahydrate MgCO3 ·10 H2O magnesium carbonate decahydrate Examples of hydrates: Write the formulas: copper(II) fluoride tetrahydrate CuF2 ·4 H2O calcium nitrate trihydrate Ca(NO3)2 ·3 H2O Write the names: MgSO4 · 7 H2O magnesium sulfate heptahydrate FeCl2 · H2O iron (II) chloride monohydrate Binary Covalent Compounds Covalent compounds are made of two NONMETAL elements sharing valence electrons. There are no ions involved!! Because there are no charges to help us write the formulas of covalent compounds, prefixes are used to indicate the number of each atom present in the formula. CO2 CO N2O SO3 is named “carbon dioxide” is named “carbon monoxide” is named “dinitrogen monoxide” is named “sulfur trioxide” The prefix, “mono” is never used for the first element in the formula! Examples of Covalent Naming Write the names: SO2 sulfur dioxide P4O10 tetraphosphorus decoxide Write the formulas: phosphorus pentachloride PCl5 dinitrogen trioxide N2O3