Naming Ternary Ionic Compounds
advertisement

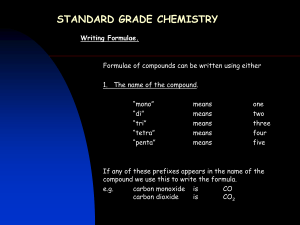
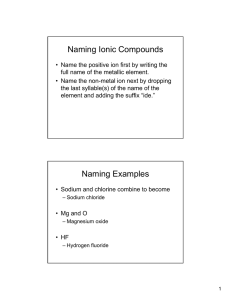
Study your notes! Quiz Ch.8 • 1. 2. 3. • 4. 5. Write the formula and name the following: Ca + Se Sr + Br Al + P Write the formula from the name: Chromium(III)oxide Cesium iodide Naming Ternary Ionic Compounds Na + NO3 NaNO3 Sodium nitrate How? (use the handout!) Polyatomic Ions (many atom ions) • Polyatomic ions are covalently bonded atoms with a collective charge. • EX: H .. N H + H+ H H+ N H H NH4+ is the ammonium ion ( +1 charge) Review the List of Polyatomic Ions Treat a Polyatomic ion as a Single ion; don’t break it up !! You know some of these ions by name. Nitrates (NO3-) Nitrites (NO2-) Phosphates ( PO4-3) Writing Formulas w/Polyatomics Li + CO3-2 Li2CO3 Mg + PO4-3 Mg3(PO4)2 NH4 + Cl NH4Cl NAMES!! Lithium carbonate Magnesium phosphate Ammonium chloride The Rules for Ternaries (very easy!!) Write the correct formula Write the name of the Cation first Write the full name for the Anion second That’s it !! Al + SO4-2 Al+3 + SO4-2 Al2(SO4)3 * use parens ( ) if you need more than 1 ion! Aluminum sulfate You have 5 min to review notes from last class. Write the formulas for : 1. Ca + N 2. Al + SO3 -2 3. Mg + phosphate Write the names for the above. Write the formula for: 1. Magnesium carbonate 2. Nickel(II)sulfate Naming Covalent Compounds ( really easy) CO2 CO carbon carbon dioxide monoxide P2O4 diphosphorous tetraoxide SO3 sulfur trioxide Covalent Compounds consist of two non-metals!! There are no charges to balance because no electrons are lost or gained. So, how do you get the ratios? Know the prefixes Prefixes: only for Covalent Cmpds. Non-metal, Non-metal combinations Mono Di Tri Tetra Penta 1 2 3 4 5 Hexa Hepta Octa Nona Deca 6 7 8 9 10 Try These !! Name the following: PO4 NO2 NO3 N2O3 P2O5 SiO2 Write the formula: Sulfur trioxide Boron dioxide Dinitrogen pentoxide Carbon monoxide Carbon tetrachloride Handout !!