Chapter 2 of "Modern Biology" Text-
advertisement

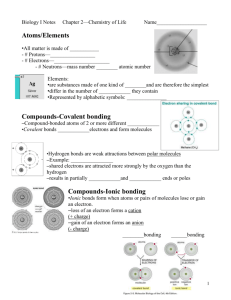
Chapter 2 CHEMISTRY 1 WHY is there a CHEMISTRY chapter in my Biology book? • Structure and function of all living things are governed by the laws of chemistry • QUESTION: What examples can you give of how chemistry is involved in biology? • Understanding the basic principles of chemistry will give you a better understanding of all living things and how they function! 2 CHEMISTRY- The science of the composition, structure, properties, and reactions of matter • Matter • is anything that has weight and takes up space • Elements • are the basic building blocks of matter that cannot be broken down by chemical means • Atoms • are the smallest units of an element that retain the element’s physical and chemical properties. • These bond together to form molecules 3 Elements • Pure substances than cannot be broken down into simpler substances • Periodic Table categorizes elements and shows trends • Currently, there are 118 elements, 92 which occur naturally 4 Biologists love CHONPS most of all! • 90% of the mass of living things are composed of combinations of 4 elements: O,C, H, & N • Throw in P and S and you can make almost any combination of organic molecules! – Carbs – Lipids – Nucleic acids – Amino acids, proteins 5 Atoms • Simplest part of an element that retains all properties of that element • Too small to see so we make up models to help us understand the structure of atoms and predict how they will act • Subatomic particles: • Neutrons have a neutral charge • Protons are positively charged • Neutrons and protons make up the nucleus • Electrons are negatively charged and orbit around the nucleus 6 2.1 From atoms to molecules Subatomic particles of atoms Normally, #e = #p = #n HOWEVER, some atoms of certain elements may have “extra” neutrons in their nucleus… …this will slightly change their atomic _________; they are called ______________ 2.1 From atoms to molecules Isotopes • Isotopes are atoms that Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. have the same atomic number but a different atomic mass because the number of neutrons differ larynx • Examples: 14C/12C, 127I, 131I thyroid gland trachea • Radioactive isotopes are useful in dating old objects, sterilizing food, imaging body organs and tissues through x-rays and killing cancer cells a. • Types of radiation can be harmful b. by damaging cells and DNA a: © Biomed Commun./Custom Medical Stock Photo; b(patient): Courtesy National Institutes of Health and/or causing cancer 8 (NIH); b(brain scan): © Mazzlota et al./Photo Researchers, Inc. Different elements are used in medical contrast imaging Iodine Barium 9 2.1 From atoms to molecules Molecules • Most elements do not exist by themselves in nature but rather like to combine with other elements • “Molecules” are made of atoms that are bonded together • Can be made of the same atom or different atoms Compounds • A molecule is formed when two or more atoms join together chemically. • Example, oxygen gas, O2 • A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Ex: WATER. 2 H atoms + 1 O atom = 1 H2O molecule • Chemical properties of compounds are often very different than the elements alone (2 gases = liquid) • “Chemical reactions”- chemical bonds can be broken, atoms can be rearranged, and new chemical bonds are formed! 11 Types of Chemical Bonds • Most bonding takes place because atoms are most chemically stable when their outermost energy levels are filled • “Octet” rule • Ex: Fluorine (7 outer e-) • Covalent bonds: – Strong bonds – Shared electrons, simulate a full outer orbital 12 Ionic Bonds • • • • Create ions Na becomes (Na+) = loses eCl becomes (Cl-)= gains an eCreate electrical charges: when + and – charges attract, ionic bonds are created 13 States of Matter • SOLID: molecules are tightly linked; little movement and definite shape • LIQUID: molecules are less tightly linked; moves more freely than solids; conforms to container • GAS: molecules are usually not attracted to one another; move very fast; fills the entire volume of a container 14 WATER • Living things are 70-80% water by weight • Most chemical rxns in living things take place in aqueous environments (either inside or outside the cells) • Water is needed to dissolve and transport nutrients, gases, etc. around us (blood, tissue fluid, saliva, sweat, etc) 15 2.2 Water and living things What are the properties of water? • Liquid at room temperature • Liquid water does not change temperature quickly • High heat of vaporization • Frozen water is less dense than liquid water • Molecules of water cling together, “cohesion” and to other polar substances “adhesion” • A good solvent for other polar (+/-) molecules Water is a covalently bonded molecule that is also POLAR (has +, - regions) • 2 Hydrogen atoms bond with an Oxygen atom at an angle • Region of the molecule where the O atom is located has a slightly neg.(-) charge, while the regions of the molecule where the two H+ atoms are have a slightly positive charge. • Oxygen has a greater “custody” of the shared electrons 17 Hydrogen Bonds • Hydrogen Bonds: negative part of the water molecule (O) forms a bind with the positive charge of the H atoms – Relatively weak, singly, but rather strong collectively – Cause H2O molecules to cling together & to other substances! 18 Water’s polarity is responsible for some of its unique properties: Cohesion• Water molecules are attracted (+/-) to other nearby water molecules; bonds them together • Surface tension = cohesive forces between water molecules are strong enough to act as if their was a "skin" in the water surface 19 Water Properties Adhesion• Water is attracted (+/-) to other substances. • meniscus = adhesive forces between water molecules and glass 20 Water Properties • Water is very important in temperature regulation - resists quick temperature changes and maintains homeostasis! • H2O has a very high specific heat- which means it can absorb or lose a large amount of heat energy before its temperature changes. • Thus, water has a moderating effect on temperatures (ex: body temp). 21 Water Properties • Water is less dense as a solid • As liquid water cools, it’s molecules slow down and come closer together, until they reach 4°C. • Below 4°C (approaching 0°C) they stop moving, and hydrogen bonds become fixed, rigid, and push apart, opening up spaces between the molecules – This is why ice floats, frozen bottles and pipes burst 22 Water is a almost-universal solvent • Water is extremely important to all living things, so the chemistry of living things often involves the study of solutions • The polar (+/-) nature of water makes it a great solvent for other polar compounds to dissolve in. • Hydrophilic or hydrophobic? • What types of substances mix well with water? 23 Know your terms • Solution: mixture in which substances are uniformly distributed in another substance – Solutions can be mixtures of liquids, solids, or gases • Solute: • Solvent: • Concentration [ ]: measurement of the amount of solute dissolved in a fixed amount of solvent • Saturated? • Aqueous solutions: 24 2.2 Water and living things Acids and bases • Acids are substances that dissociate and release hydrogen ions (H+) • Ex: HCl H+ + Cl- • Bases are substances that take up hydrogen atoms or release hydroxide ions (OH-) Ex: NaOH Na+ + OH- Dissociation: molecules come apart when enough “pull” is exerted • Pure water dissociates into H+ and OH- equally (hydrogen and hydroxide) H2O H+ + OH- Acids and Bases – the pH scale • Acidity and Alkalinity is a measure of the relative amount of OH- and H+ ions in a solution! • pH= measure of how many H+ ions are in a solution • Pure water has equal OH- and H+ ions in solution; pH of 7.0 • Acidic solutions have H+ > OH- ions – pH is below 7.0 – Sour • Basic solutions have H+ < OH- ions – pH is above 7.0 – slippery and bitter 26 Working scale is between 0 and 14 with 7 being neutral A pH below 7 is acidic and above 7 is basic The concentration of ions between each whole number is a factor of 10 Buffers • …are chemical substances that neutralize small amounts of either an acid or a base added to a solution • Most chemical rxn’s in living organisms are controlled by pH, therefore... • Buffers are very important for homeostasis. – If blood pH drops below 7.0 (acidosis), it could be fatal – If blood pH goes above 7.7 (alkalosis), it could be fatal • If our blood did not contain a buffering system, we would not be able to drink and eat acidic/basic foods! Bicarbonate 28 Organic Compounds • All compounds discovered can be classified into two broad categories: inorganic and organic • "Organic" = • The compounds of life consist of primarily 6 elements: "CHONPS" 29 Chemistry of CARBON is the chemistry of LIFE! • Carbon forms the “backbone” (framework) of all organic molecules • C has four e- in its outermost energy level, but needs 8 to fill it, so it readily forms covalent bonds! 30 Carbon, the basis for life • Carbon likes to bond, with other atoms and with itself • single bonds• double bonds• triple bonds31 Simple & Complex Molecules • Molecules are often built up from smaller, simpler molecules: MONOMERS • Monomers bond together to produce: POLYMERS • Large polymers are called: MACROMOLECULES • Dehydration reaction – the removal of water that allows subunits to link together into larger molecules • Hydrolysis reaction – the addition of water that breaks larger molecules into their subunits 32 There are Four Major Classes of Organic Compounds: CARBOHYDRATES (= ENERGY) • - The most abundant organic compounds in nature - C:H:O = approx. 1:2:1 Monosaccharides - simple sugars; "building blocks of all carbs" • C6H12O6 Three main monosaccharides: • glucose- main source of energy for cells • fructose- sugar in fruits and honey (the sweetest monosaccharide) • galactose- sugar in milk and yogurt 33 Carbohydrates, cont’d Disaccharides - two monosaccharides bonded together by dehydration rxn’s (form glycosidic bonds) Examples: • glucose + fructose = sucrose (common table sugar) •glucose + galactose = lactose (major sugar in milk) 34 2.4 Carbohydrates What are “complex” carbohydrates? • Polysaccharides are made of many carbon rings • Cell stores energy it doesn’t need by converting monosaccharides into disaccharides/polysaccharides • Starch is the storage form in plants • • Glycogen- is the storage form in animals – stored in liver and muscles- once storage is full, glycogen turns to fat! The Role of Enzymes in Starch Digestion (Amylase) • Monosaccharides- soluble in water & CAN pass through cell membrane by diffusion • Disaccharides- are soluble in water and CANNOT pass through the cell membrane (too BIG!) – when a cell needs energy, disaccharides are broken down into its monomers by hydrolysis! • Polysaccharides- are NOT soluble in water and CANNOT pass through the cell membrane without a series of hydrolysis rxn’s! 36 2.5 Lipids What are lipids? • Molecules that do not dissolve in water • Used as energy storage molecules, insulation, cushion • Found in cell membranes • Found as fats and oils, waxes, phospholipids and steroids 2.5 Lipids How are fats and oils different? • Fats – Usually animal origin – Solid at room temperature – Function as long-term energy storage, insulation from heat loss and cushion for organs • Oils • Usually plant origin • Liquid at room temperature 2.5 Lipids What is the structure of fats and oils? • TRIGLYCERIDES: A glycerol molecule and 3 fatty acid tails 2.5 Lipids Understanding fats when reading a nutrition label • Recommendation for total amount of fat for a 2,000 calorie diet is 65g • Be sure to know how many servings there are • A % DV of 5% or less is low and 20% or more is high • Try to stay away from trans fats • Would you eat the food on the right? Why or why not? Trace elements: Are required by an organism in only minute quantities Make up the remaining 4% of living matter Table 2.1 • The effects of essential element deficiencies Figure 2.3 (a) Nitrogen deficiency (b) Iodine deficiency 2.5 Lipids What is the structure of a phospholipid? Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. • The structure is similar to a triglyceride. • One fatty acid is replaced by a polar phosphate group. • Phospholipids are the primary components of cellular membranes. polar head inside cell nonpolar tails outside cell a. Phospholipid structure Figure 2.19 Structure of a phospholipid. b. Membrane structure 2.5 Lipids What are steroids? • A lipid • Structure is four fused carbon rings Important steroids: Cholesterol, testosterone, estrogen, progesterone, cortisol 2.6 Proteins What are proteins? • Large and often complicated molecules • Make up skin, muscles, pigments, antibodies, hormones & enzymes • Hundreds of thousands of different kinds in each cell • Mostly C,H,O, & N • Composed of amino acid monomers (20 AA groups in total) • Can denature, change in shape, that causes loss of function 2.6 Proteins What do amino acids look like? 2.6 Proteins What do the levels of organization look like? • Support: keratin (hairs, nails) , collagen (ligaments, tendons, skin) • Transport: channel proteins allow certain molecules through cell membranes; hemoglobin transports oxygen (RBC) • Defense: antibodies are protein made by WBC • Hormones: regulate cell metabolism and growth (ex: insulin) • Motion: muscle tissue made of contractile proteins (actin & myosin) • Enzymes: catalyze chemical reactions in cells 48 ENZYMES are important proteins • Many chemical reactions in living cells (and organisms) are regulated by ENZYMES • Enzymes are globular proteins in living systems that mediate metabolic reactions (make and break chemical bonds) – Metabolism: the series of energy exchanges and chemical reactions that occur in living systems (cells, organisms) – catabolic activities = breakdown of larger molecules into smaller; AB ==> A + B – anabolic activities = synthesis of larger molecules from smaller ones; A + B ==> AB 49 Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the “Normal” or “Slide Sorter” views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer. 2.7 Nucleic acids What are nucleic acids? • Made of nucleotide subunits • Function in the cell to make proteins • Directs traits and behaviors • Includes RNA and DNA NUCLEIC ACIDS • Nucleic acids are long chains (polymers)of nucleotides • Nucleotides are the monomers of nucleic acids Each nucleotide includes a nitrogenous base, 5-C sugar, and phosphate group 52 DNA: Deoxyribonucleic acid • Contains genetic information • Composed of: 1) Deoxyribose sugar 2) Phosphate group 3) 4 different “base" groups: Adenine, Guanine, Cytosine, and Thymine 53 RNA: Ribonucleic acid • Stores and transfers genetic information for making proteins from nucleus to ribosomes • Like DNA, RNA is composed of nucleotides: 1) Ribose sugar 2) Phosphate group 3) 4 different “base" groups: Adenine, Guanine, Cytosine, and Uracil • DNA IS DOUBLE STRANDED AND RNA IS SINGLE STRANDED! 54 Summary of the macromolecules