Rocks and Mineral Continued
advertisement

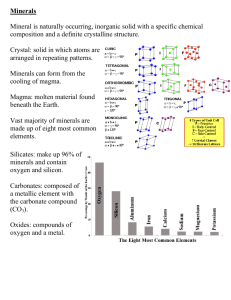
Metamorphic rocks are rocks that have "morphed" into another kind of rock. These rocks were once igneous or sedimentary rocks. The rocks are under tons and tons of pressure, which fosters heat build up, and this causes them to change underground. As a result, most of the thousands of rare minerals known to science occur in metamorphic rocks. The presence of mineral layers, called foliation, is important to observe when identifying a metamorphic rock. Schist Parent- Shale or Phyllite Gneiss Parent- Granitic and volcanic rocks Slate Parent- Shale Phyllite Parent- Mudstones and shale Marble Parent- Limestone Quartzite Parent- Quartz sandstone Physical Properties- Properties used to visually identify a specific mineral Color Luster Transparency Crystal systems Cleavage Fracture Hardness Specific Gravity Streak Color is the first thing someone notices when they view a mineral. Color is also one of the big reasons that attract people to minerals. Color in minerals is caused by the absorption, or lack of absorption, of various wavelengths of light. Many minerals come in different colors and some minerals' colors are identical to other minerals' colors. Ex. Quartz can come in pink, purple, white, golden yellow or smoky grey. Luster is a description of the way light interacts with the surface of a crystal. Types of Luster- 2 main categories Metallic - the look of metals (shiny) Ex. Gold, Silver, Galena, Pyrite Non-metallic- not shiny Ex. Sulfur, Feldspar, Talc Adamantine-Clear and Brilliant like a diamond (most gems) Dull- a non-reflective surface of any kind Earthy- the look of dirt or dried mud Pearly- the look of a pearl Waxy- the look of wax Fibrous- the look of fibers Greasy- the look of grease Resinous- the look of resins such as dried glue or chewing gum Vitreous-the most common luster, it simply means the look of glass Transparent- light enters and exits the surface of the substance in relatively undisturbed fashion. Can see all visible light and can distinguish an object when looking through it. Ex. Diamond Translucent- light enters and exits the surface of the substance in relatively undisturbed fashion. Can see light through it, but can not clearly distinguish an object while looking through it. Ex. Quartz Opaque- If the light can not even penetrate the surface of the substance. Ex. Feldspar 1. 2. 3. 4. 5. 6. 7. A mineral has flat sides called faces. These faces are flat and join at different angles. The joined faces make a certain shape. Cubic- Crystals grow in the shape of a cube Tetragonal- square bottom and top face with a rectangular body. Orthorhombic- a rectangular prism with a rectangular base Monoclinic-They form a rectangular prism with a parallelogram as its base. TriclinicHexagonal- face forms a hexagon Trigonal-similar to a cube that has been compressed to one side. › Crystal cleavage is a smooth break › › › › › producing what appears to be a flat crystal face. Cleavage is reproducible, meaning that a crystal can be broken along the same parallel plane over and over again All cleavage must parallel All cleavage planes of a mineral must match that mineral's crystal system The same mineral will always, always have the same cleavage Cleavage occurs in minerals that have specific planes of weakness Ex. Fluorite= Octahedral Cleavage, Calcite= Rhombohedral, Halite=cubic The way a Rock Breaks Types of Fracture Conchoidal- smoothly curved fracture that looks like a piece of chipped or broken glass. Sometimes described as a clam-shell fracture. Ex. Quartz, Obsidian Uneven- basically self explanatory, the fracture does not have a pattern and can not be identically repeated Ex. Anhydrite Jagged- has sharp points or edges that catch on a finger that's rubbed across the surface. Ex. Metals such as Copper Hardness- measure of the strength of the structure of the mineral relative to the strength of its chemical bonds. Minerals with small atoms, packed tightly together with strong covalent bonds throughout tend to be the hardest minerals. The softest minerals have metallic bonds or even weaker van der Waals bonds as important components of their structure Hardness can be tested through scratching. A mineral can only be scratched by a harder substance. Use Mohs Hardness Scale Mohs Hardness Scale 1. Talc 2. Gypsum 3. Calcite 4. Fluorite 5. Apatite 6. Orthoclase 7. Quartz 8. Topaz 9. Corundum (ruby and sapphire) 10. Diamond Specific Gravity- measure of the density of a mineral. Non-metallic minerals tend to be of a low density Metallic minerals tend to be of higher density HEFTING- To use specific gravity, hold a mineral of unknown SG in one hand and in the other hand a mineral of known SG preferably one near the average of 2.75 and of the same size as the unknown mineral; then compare. Ex. Galena vs. Quartz Streak is actually the color of the powder of a mineral. Streak is closely related to color, but is a different property because the color of the mineral may be different than the color of the streak The proper way to test for streak is to rub a mineral across a tile of white unglazed porcelain and to examine the color of the "streak" left behind. Two minerals that have similar outward color may have different colors when powdered. Ex. the minerals hematite and galena can be confused when both have a gray color. However, hematite's streak is reddish-brown, while galena's streak is lead gray. When two tectonic plates collide one may be pushed down into the mantle. It heats and melts Magma that is made will have different chemical composition depending on where it comes from. Magmas from Continental Crust - rich in silicon and aluminum Magmas from oceanic crust - rich in iron and magnesium Composition determines which rocks will be made when magma cools N.L. Bowen - showed that cooling magmas produce minerals in a predictable order Two sides - Iron (Fe) rich side and a calcium / sodium (Ca / Na) rich side. Magma types MAFIC Basalt Intermediate Andesite FELSIC Rhyolite Olivine - First Fe rich mineral to form - in tetrahedra shape - easily weathered on the surface Pyroxenes - Tetrahedra link in long chains also weather easily Amphiboles - double strands made from linking pyroxenes Biotite Micas - amphibole chains make sheets Calcium/Sodium Feldspar - from the Ca/Na side like olivine but Al is inside the tetrahedra instead of Silicon Potassium Feldspars - as temp cools Ca and Na replace Al inside the tetrahedron Quartz - very low temperatures - little left in magma except Si and O -three dimensional networks