Acid-Base Titration - Answers, Sources of Error
advertisement
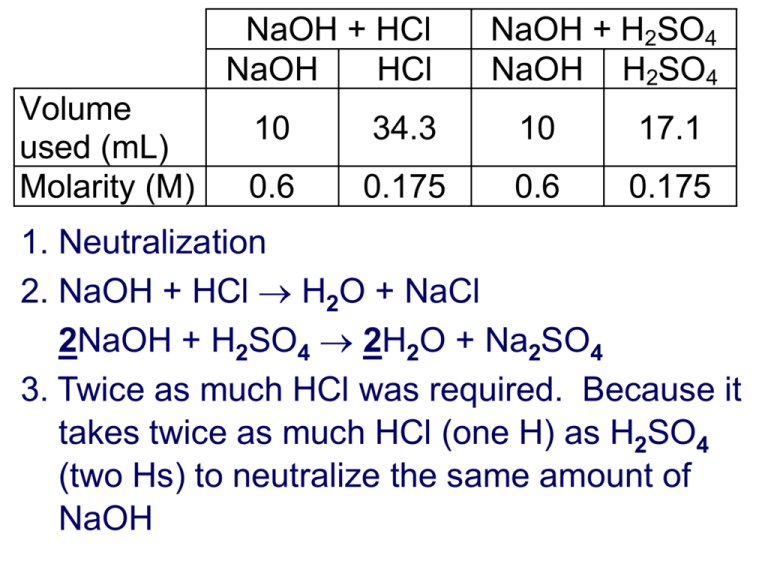
NaOH + HCl NaOH HCl Volume used (mL) Molarity (M) NaOH + H2SO4 NaOH H2SO4 10 34.3 10 17.1 0.6 0.175 0.6 0.175 1. Neutralization 2. NaOH + HCl H2O + NaCl 2NaOH + H2SO4 2H2O + Na2SO4 3. Twice as much HCl was required. Because it takes twice as much HCl (one H) as H2SO4 (two Hs) to neutralize the same amount of NaOH 4. H2SO4(aq) + 2NaOH(aq) 2H2O + Na2SO4(aq) # L H2SO4= 0.010 L NaOHx0.60 mol NaOH 1 mol H2SO4 L H2SO4 x x L NaOH 2 mol NaOH 0.175 mol H2SO = 0.01714 L = 17.1 mL 5. #H x MA x VA = #OH x MB x VB Titration problems 1. What volume of 0.10 mol/L NaOH is needed to neutralize 25.0 mL of 0.15 mol/L H3PO4? 2. 25.0 mL of HCl(aq) was neutralized by 40.0 mL of 0.10 mol/L Ca(OH)2 solution. What was the concentration of HCl? 3. A truck carrying sulfuric acid is in an accident. A laboratory analyzes a sample of the spilled acid and finds that 20 mL of acid is neutralized by 60 mL of 4.0 mol/L NaOH solution. What is the concentration of the acid? 4. What volume of 1.50 mol/L H2S will neutralize a solution containing 32.0 g NaOH? Titration problems 1. (3)(0.15 M)(0.0250 L) = (1)(0.10 M)(VB) VB= (3)(0.15 M)(0.0250 L) / (1)(0.10 M) = 0.11 L 2. (1)(MA)(0.0250 L) = (2)(0.10 M)(0.040 L) MA= (2)(0.10 M)(0.040 L) / (1)(0.0250 L) = 0.32 M 3. Sulfuric acid = H2SO4 (2)(MA)(0.020 L) = (1)(4.0 mol/L)(0.060 L) MA = (1)(4.0 M)(0.060 L) / (2)(0.020 L) = 6.0 M 4. mol NaOH = 32.0 g x 1 mol/40.00 g = 0.800 (2)(1.50 mol/L)(VA) = (1)(0.800 mol) VA= (1)(0.800 mol) / (2)(1.50 mol/L) = 0.267 L Titration summary • For titrations we use the formula: #H x MA x VA = #OH x MB x VB Or NA x VA = NB x VB • NA is the combination of MA and #H • N is also known as normality • You can think of it a neutralizing power • We will stick with the first equation, you do not have to know N or what it stands for • This is a simplification of stoichiometry. We could get the same answer by working with moles (n = MV) and by using the balanced chemical equation Titration showdown • Titration competition (best with a burette): find the concentration of an H2SO4 solution – it could be anywhere from 0-18 mol/L • You will use the NaOH that you prepared two weeks ago and your vast knowledge of titration procedures and formulas. • The wining team, is the team closest to the correct value. • The only restriction is : don’t put base in the burette – only acid. • • • • • • • • Sources of error NaOH was not exactly 0.10 mol/L Calculation errors (e.g. converting mL to L) Not rinsing and drying the beaker for the acid Over titrating (ideally, 2 titrations should be done – one to get a rough estimate, and one to get the exact value). Acid or base left on the side of the flask or on the tip of the burette (for greater precision, water is used to rinse the tip of the burette). Errors reading volumes. Using pipette (10 mL is measured from 0 mL to 10 mL, not from 10 mL to empty) Using 10 mL of base vs. 25-50 mL For more lessons, visit www.chalkbored.com





