Changing Atomic Model
advertisement
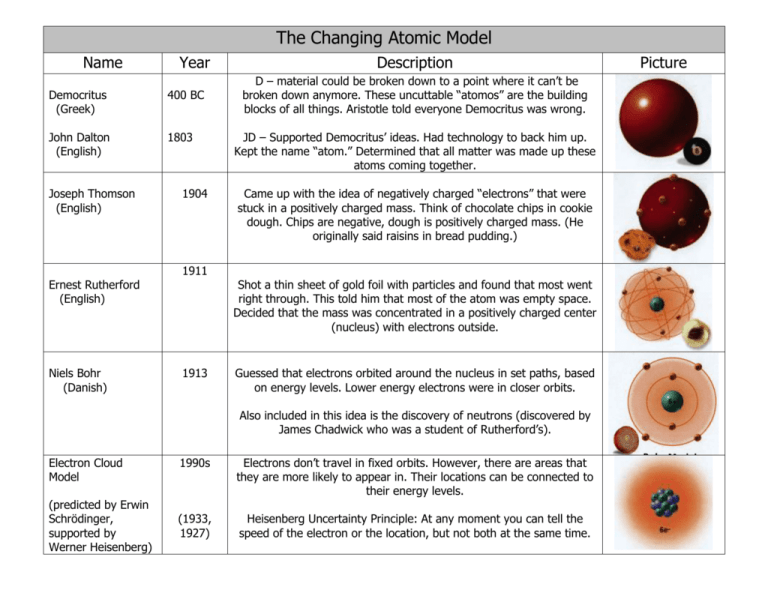
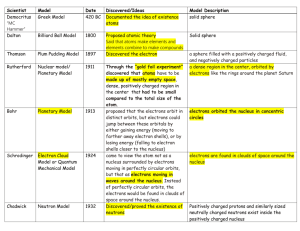
The Changing Atomic Model Name Year Democritus (Greek) 400 BC John Dalton (English) 1803 Joseph Thomson (English) 1904 Description D – material could be broken down to a point where it can’t be broken down anymore. These uncuttable “atomos” are the building blocks of all things. Aristotle told everyone Democritus was wrong. JD – Supported Democritus’ ideas. Had technology to back him up. Kept the name “atom.” Determined that all matter was made up these atoms coming together. Came up with the idea of negatively charged “electrons” that were stuck in a positively charged mass. Think of chocolate chips in cookie dough. Chips are negative, dough is positively charged mass. (He originally said raisins in bread pudding.) 1911 Ernest Rutherford (English) Niels Bohr (Danish) Shot a thin sheet of gold foil with particles and found that most went right through. This told him that most of the atom was empty space. Decided that the mass was concentrated in a positively charged center (nucleus) with electrons outside. 1913 Guessed that electrons orbited around the nucleus in set paths, based on energy levels. Lower energy electrons were in closer orbits. Also included in this idea is the discovery of neutrons (discovered by James Chadwick who was a student of Rutherford’s). Electron Cloud Model (predicted by Erwin Schrödinger, supported by Werner Heisenberg) 1990s Electrons don’t travel in fixed orbits. However, there are areas that they are more likely to appear in. Their locations can be connected to their energy levels. (1933, 1927) Heisenberg Uncertainty Principle: At any moment you can tell the speed of the electron or the location, but not both at the same time. Picture