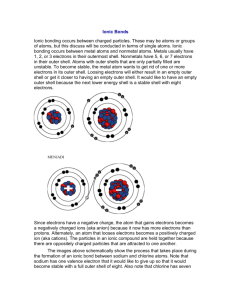
Review on Atoms Please describe or define what a ……. Proton
advertisement

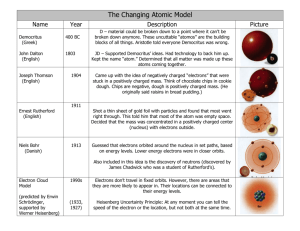
Review on Atoms Please describe or define what a ……. ProtonElectronNeutronNucleusCompound elementAtomIsotopeValence ElectronIonPlease fill in the blank Atomic Number= = . Atomic Mass rounded = . Mass Number- Protons = . How many electrons does the first energy shell hold? How many electrons does the second energy shell hold? How many electrons does the third energy shell hold? Use the word bank to answer the following questions Democritus Neils Bohr 1. John Dalton compounds J.J. Thomson Ernest Rutherford electron cloud Greek philosopher who asked “could matter be divided into smaller and smaller pieces forever?” 2. named the smallest piece of matter “atomos” meaning “not to be cut” 3. said elements are composed of atoms. Atoms of the same element are exactly alike and atoms of different elements are different. are formed by joining two atoms of two or more elements. 4. is responsible for the Plum Pudding Model which stated atoms are made from a positively charged substance with negatively charged electrons scattered about the atom 5. reasoned that all of the atom’s positively charged particles were contained in the nucleus. The negatively charged particles were scattered outside the nucleus in the . 6. placed each electron on a specific energy level. He is also famous for the Model. Atomic # __________ Atomic Mass __________ _______ Mass Number __________ _____ _____ # of Protons __________ ______ ____ # of Neutrons __________ ____ # of Electrons __________ _______ ___ _________ ________ __ # of Valence Electrons ___ ______