BONDING AND NOMENCLATURE REVIEW
advertisement

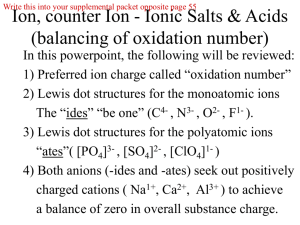
BONDING AND NOMENCLATURE REVIEW BONDING State the octet rule. Complete the table below. Element symbol Formula for ion 1. Sr Sr2+ 2. P # of electrons gained or lost Name of ion Anion or Cation? strontium ion 3 electrons gained 3. Br 4. 5. Cs oxide ion 1 electron lost Complete the table below. Formula and charge for ion 1. SO32- Name of ion 2. PO433. ammonium ion 4. CO325. chlorite ion 6. chromate ion What is the difference between an ionic bond and a covalent bond? State whether the following compounds would contain ionic bonds, covalent bonds, or both. a. KF________________ b. Br2_______________ c. N2Cl4_______________ d. P2O5_______________ e. FeS_______________ f. NH4OH_____________ g. H2SO4_____________ h. CBr4_______________ i. Mg3(PO4)2____________ Draw all of the resonance structures and name the 3-dimensional shape of this molecule: CO32- For each of the following, draw the lewis structure and name the 3-dimensional shape (tetrahedral, pyramidal, bent, linear, or triangular planar). State whether the molecule is polar or nonpolar. If it is polar, then indicate polarity with an arrow or with the appropriate symbols for partial charges. PCl3 SO42- CO2 H 2S NO2- SO32- NOMENCLATURE Write the correct chemical names or formulas for the following acids: HF H2CO3 H2SO3 HCN nitrous acid chloric acid Complete the chart below. (Note: ionic compounds and molecular compounds are mixed together!) Formula Name Ca(OH)2 Sodium phosphate Nitrogen trichloride Pb3(PO4) 2 Mn3N2 NBr3 Cobalt (II) nitrite Zinc bicarbonate Nitrogen monoxide AgNO3 PCl3 MgCrO4 CsI Strontium sulfate Ba(ClO3) 2 Phosphorus triiodide Manganese (II) oxide SnF2 Potassium dichromate Lithium sulfite Al2 (CO3) 3