CHM 50- Class activity - Chapter 5
advertisement

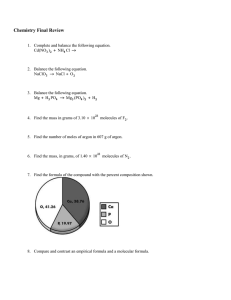
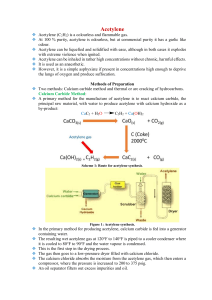
CHM 50 Homework – Chapter 5-part 2 Name Due Monday 10/13/14 1. If I place 3 moles of N2 and 4 moles of O2 in a 35 L container at a temperature of 250 C, what will the pressure of the resulting mixture of gases be? 2. 27.5 g of fluorine gas is collected over water. If the gas is at 5.00˚C, what is the volume of the fluorine gas if the total pressure of the water and the fluorine gas is 32.0 kPa? 3. A tank contains 480.0 grams of oxygen and 80.00 grams of helium at a total pressure of 7.00 atmospheres. Calculate the following. a) How many moles of O2 are in the tank? b) How many moles of He are in the tank? c) Total moles of gas in tank. d) Mole fraction of O2. e) Mole fraction of He. f) Partial pressure of O2. g) Partial pressure of He. 4. Calculate the mass of hydrogen peroxide needed to obtain 0.460L of oxygen gas at STP. 2H2O2 (aq) 2H2O (l) + O2 (g) 5. a. Acetylene gas (C2H2) undergoes combustion to produce carbon dioxide and water vapor. Write a balanced chemical equation b. How many liters of C2H2 are required to produce 75.0 L of CO2? c. What volume of H2O is produced? d. What volume of O2 is required? 6. A 3.25 gram sample of solid calcium carbide (CaC2) reacts with water to produce acetylene gas (C2H2) and aqueous calcium hydroxide. If the acetylene was collected over water at 17ºC and 740.0 mm Hg, how many milliliters of acetylene were produced? 7. When chlorine is added to acetylene, 1, 1, 2, 2-tetrachloroethane is formed: 2Cl2(g) + C2H2(g) C2H2Cl4(g) How many liters of chlorine will be needed to make 75.0 grams of C2H2Cl4 at 1.90 atm and 30.0 oC? 8. A sample of hydrogen effuses through a porous container 7.50 times faster than an unknown gas. What is the molecular mass of this unknown gas? 9. Which gas would effuse faster: Ne or CO2? How much faster? 10. Use the van der Waals equation and the ideal gas equation to calculate the volume of 1.000 mol of neon gas at a pressure of 500.0 atm and a temperature of 355.0 K. Explain why the two values are different. (For Ne: a = 0.211 L2 atm/mol2 and b = 0.0171 L/mol)