a - BrainMass
advertisement
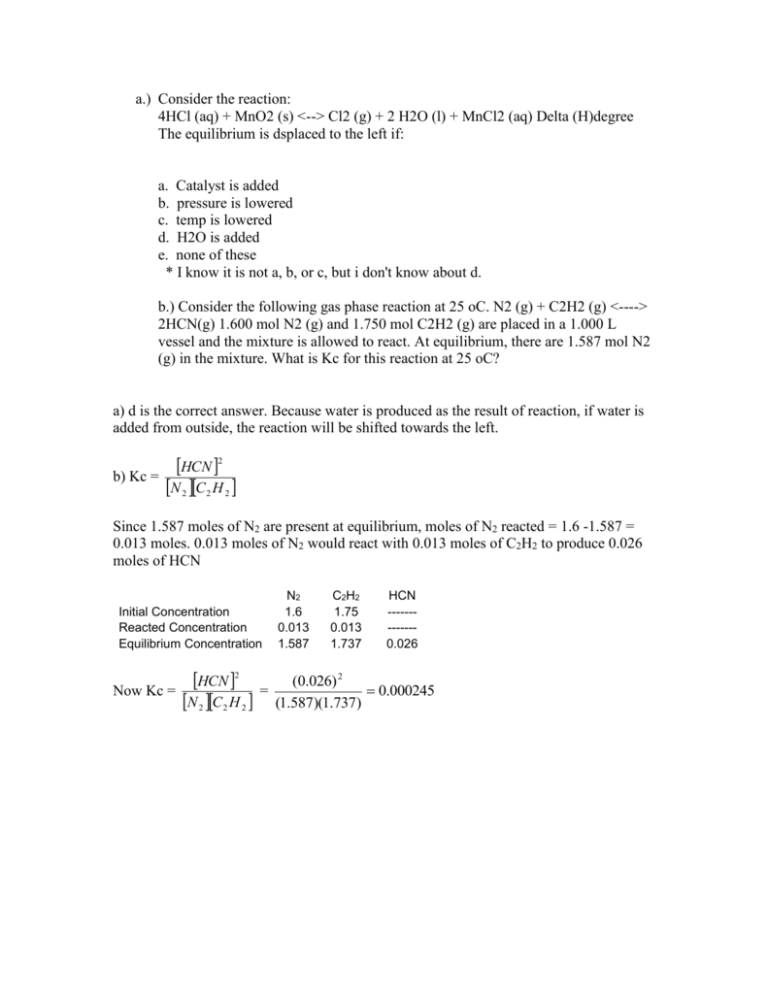
a.) Consider the reaction: 4HCl (aq) + MnO2 (s) <--> Cl2 (g) + 2 H2O (l) + MnCl2 (aq) Delta (H)degree The equilibrium is dsplaced to the left if: a. Catalyst is added b. pressure is lowered c. temp is lowered d. H2O is added e. none of these * I know it is not a, b, or c, but i don't know about d. b.) Consider the following gas phase reaction at 25 oC. N2 (g) + C2H2 (g) <----> 2HCN(g) 1.600 mol N2 (g) and 1.750 mol C2H2 (g) are placed in a 1.000 L vessel and the mixture is allowed to react. At equilibrium, there are 1.587 mol N2 (g) in the mixture. What is Kc for this reaction at 25 oC? a) d is the correct answer. Because water is produced as the result of reaction, if water is added from outside, the reaction will be shifted towards the left. b) Kc = HCN 2 N 2 C 2 H 2 Since 1.587 moles of N2 are present at equilibrium, moles of N2 reacted = 1.6 -1.587 = 0.013 moles. 0.013 moles of N2 would react with 0.013 moles of C2H2 to produce 0.026 moles of HCN Initial Concentration Reacted Concentration Equilibrium Concentration Now Kc = HCN 2 N 2 C 2 H 2 = N2 1.6 0.013 1.587 C2H2 1.75 0.013 1.737 HCN ------------0.026 (0.026) 2 0.000245 (1.587)(1.737)











