NOTE
advertisement

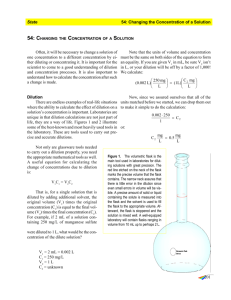
Lab problems/calculations....more practice About printing: If this is printed in portrait format, some conversion factor ratios may not display properly. So, set left/right margins to the smallest possible or (better) print in landscape format. In the first five lab sessions you have seen several different kinds of problems that are common in lab work: e.g. metric conversions, dimensional analysis, dilution work, expressions of change, concentration expressions. These are not mutually exclusive; a single problem may involve two or more of these. The problems shown here are examples of the types of problems that you might see on a lab exam, quiz, or in future courses. These particular problems are not important, but understanding how to solve them is important. These require that you know what you were told (in the first lab period) you would be responsible for: a. knowing the metric system basic units and prefixes, b. knowing the foot-pound system conversions (inches to feet, ounces to quarts, etc.), c. knowing three (specifically 3) conversion factors for moving between the two systems, d. knowing how to use the dimensional analysis method. In working such problems you would be required to show all steps with correct units in every step. It is important to show units in every step and to show every step; failure to do so increases the likelihood that you'll make careless mistakes. And careless errors cost points too. 1. Red blood cells (RBC) have the shape of a biconcave disk. As seen through the microscope they appear as pink circles, since the disks lie flat on the microscope slide. The typical RBC diameter is about 8 micrometers, and there are about 5 billion RBC in one milliliter of blood. If all of the RBC in 1 milliliter of blood were placed edge to edge in a straight line, how long would that line be? Express the answer in feet. (ANSWER) 2. Give the answer in two decimal places. In the typical human body there are about 2.35 X 1013 red blood cells (RBC), and one milliliter of blood contains about 5 billion RBC. Calculate how many pints of blood are in the typical human body. (ANSWER) 3. Round the answer to the nearest whole number. The energy content of one pound of fat is 3500 calories. Sam plans to lose 10 pounds of body fat by walking regularly. By walking at the rate of 8 kilometers per hour he will burn 300 calories per hour. How many miles (total) must he walk to lose the 10 pounds? (ANSWER) 4. Sam's body contains 4.0 liters of blood. Because of an injury he is losing blood (through a cut) at the rate of 3 ounces per minute. Since he can't stop the bleeding or drive himself to the hospital, he called 911. If his body's blood volume decreases by 40%, he will lapse into a coma. How much time do the paramedics have to reach him before he loses consciousness? Round the answer to the nearest whole minute. (ANSWER) 5. Describe how to prepare 50 mL of a 0.25 M solution of KCl. (ANSWER) 6. If 35 mL concentrated HCl (hydrochloric acid, 11.65 M) is diluted with water to 500 mL, what is the concentration of the new solution? [NOTE: when mixing water and concentrated acids, always add the acid to water, not water to acid!! Adding water to acid may produce a violent reaction with splashing.] (ANSWER) 7. Round the answer to the nearest integer. The treatment for a certain canine illness is drug "ABC" at a dosage of 2 mg per kilogram of body weight. The bottled drug comes from the pharmaceutical company to veterinarians at a concentration of 1 g per 100 mL of solution. FooFoo, the pooch, weighs 21 pounds. How many milliliters of this ABC solution must the vet give to FooFoo to treat the illness? (ANSWER) 8. An exercise requires you to prepare exactly 1 liter of a glucose solution at a concentration of 21.6 micrograms per milliliter. To do this you are given water, whatever glassware you need (pipets, graduated cylinders, beakers, e.g.) and a stock glucose solution at a concentration of 4.7 milligrams per milliliter. How do you prepare the required solution? Carry one decimal place throughout. (ANSWER) 9. Which one of the following solutions has the greatest solute concentration? In each the solvent is water and the solute is sodium chloride. a. 1.5% (w/v); b. 15 microgram/mL; c. 150 mg/L; d. 1.5 g/L; e. 0.15 mg/dL; f. 0.15 oz./quart; g. 1.5 X 10-3 oz./gallon (ANSWER) 10. One bottle of salt solution is marked "30 grams per 100 ml of solution." (The solvent is water.) Another bottle is marked "30 grams per 100 mL of water. " Is this just two ways of expressing the same thing? If not, what's the difference? (ANSWER) 11. Given: a 0.8 M solution of alanine (an amino acid). Prepare 30 mL of a 1:10 dilution of this alanine solution. [This is a parts problem.] (ANSWER) 12. In some kinds of lab work it is necessary to perform serial dilutions. That means dilution of a dilution, perhaps several times. Example: A virologist was trying to determine how many H1N1 bird flu virus particles were necessary to initiate an infection. She had a purified preparation of the virus, a suspension containing 5 X 105 virus particles per milliliter. (Notation: Virus particles are too large to dissolve, but they can be suspended in water or buffer solution.) The virologist diluted this original suspension 1:10, then diluted that suspension again 1:10, and then diluted that suspension 1:10 again. Then she squirted 1 mL of each diluted suspension into the airways of test animals and watched for development of respiratory symptoms. In fact, she exposed several individual animals to each of the three diluted suspensions, since the result from a single animal might not be representative. She knows that the treatments need to be replicated if the results are to be reliable. Your questions: a. What is the virus particle concentration in each of the three diluted suspensions? b. How does she prepare these three suspensions to be tested? (ANSWER) SOLUTIONS BELOW Solution to problem 1: What's given? There are 5 X 109 RBC per mL of blood. Note that this is scientific notation for 5 billion. You're also told that each RBC is 8 micrometers in diameter. What must you do? Line up, edge to edge, all the RBC in one ml. Then express the length of that line in feet. 5 X 109 RBC mL X 1 mL X X __1 m__ X 106 μm 8 μm = RBC 4 X 1010 μm Then: 4 X 1010 μm 39.37 in. X m 1 ft._ = 12 in. 1.31 X 105 ft. Note that the system-to-system conversion factor required for this problem is 39.37 inch/meter; that’s from lab #1. As noted in that lab, there are three specific system-to-system conversion factors that we are required to use in working problems in this course. back to problem 1 Solution to problem 2: What's given? 2.35 X 1013 RBC (total) in the body. One ml contains 5 X 109 RBC. What's asked for? Volume of blood in the body, expressed in pints.... In other words, what volume of whole blood corresponds to this total number of RBC? 2.35 X 1013 RBC X 1 mL 5 X 109 RBC X __1 L___ X 1000 mL 1.057 qt. X L 2 pt. = qt. 9.94 pints Note that this problem involves another chain operation. From left to right, the units cancel: RBC, then mL, then L, then qt., leaving the desired units, pints. Also note that the system-to-system conversion factor required for this problem is 1.057 quart/liter; that’s from lab #1. As noted in that lab, there are three specific system-to-system conversion factors that we are required to use in working problems in this course. back to problem 2 Solution to problem 3: He wants to lose 10 lbs. So, we need to know how many miles of energy-burning walking is equivalent to 10 pounds. Start with the 10 pounds of fat. Then use the appropriate conversion factors to get to "miles", the units that are asked for. 10 lb. fat X 3500 cal X lb. fat 1 hr. X 300 cal. 8 km X 1000 m X km km 39.37 in. X m __1 ft. X 12 in. 1 mile__ = 5280 ft. 580 miles Note that the system-to-system conversion factor required for this problem is 39.37 in./m; that’s from lab #1. As noted in that lab, there are three specific system-to-system conversion factors that we are required to use in working problems in this course. back to problem 3 Solution to problem 4: The paramedics have to arrive before his blood volume decreases 40%. Total blood volume is 4 liters. A 40% decrease is: 4 liters X 0.4 = 1.6 L Now the problem is: How long will it take for him to lose 1.6 L? The rate of blood loss is given; that's a conversion factor: 3 oz./minute 1.6 L X 1.057 qt. X L 2 pt. qt. X 16 oz. pt. X 1 minute = 3 oz. 18 minutes Note the use of the proper system-to-system conversion factor (qt./L), as required in the lab #1 instructions. back to problem 4 Solution to problem 5: 0.25 M means 0.25 molar. That’s a concentration expression, meaning 0.25 moles of KCl per liter of solution. KCl is a white crystalline salt. You need to use a balance to measure (“weigh out” is the common expression) the required mass of KCl. The balance is calibrated in gram units, not moles. So, consult the periodic table of the elements to find that KCl weighs 74.5 g per mole. 0.25 mole X 74.5 g = 18.6 g That’s the mass (weight) of KCl equivalent to 0.25 mole of KCl. L mole 18.6 g KCl dissolved in enough water to make exactly 1 L of solution would be 0.25 M. But the problem asks for preparing only 50 mL. Note that in practical lab work, it would be wasteful to prepare a larger volume of material than is needed. And when hazardous materials must be used (KCl is not), it is prudent to minimize the amounts handled. 18.6 g X L __1 L__ X 1000 mL 50 mL = 0.93 g KCl This means that 0.93 g KCl per 50 mL of solution = 0.25 M. You can check it by working backward-Watch the units. 0.93 g X 50 mL 1000 mL X L 1 mole = 74.5 g 0.25 mole = L 0.25 M Now, to actually prepare the solution: Place 0.93 g KCl crystals into a 50 mL volumetric flask and add enough water, about 30 mL (but less than 50 mL), to dissolve the salt completely. Then slowly add more water to reach the mark (50 mL) on the neck of the flask. [Recall that you used a volumetric flask in the osmosis lab to prepare a 1 M sucrose solution.] back to problem 5 Solution to problem 6: 11.65 mole X L ___1 L__ X 1000 mL 35 mL = 0.408 mole HCl This is the amount of HCl in the 35 mL. That same amount will be in the new 500 mL volume. Therefore..... 0.408 mole X 500 mL 1000 mL = L 0.816 mole = L 0.82 M (rounded) Alternate procedure, using the C1V1 = C2V2 relation. This formula may be used since this is a simple dilution problem. Note that the total amount of HCl (solute here) does not change upon dilution. The concentration of HCl will change, but the amount (mass) of HCl will not change. C1 = 11.65 M (original concentration) V1 = 35 mL (original volume) C2 = X (new concentration) V2 = 500 mL Then 11.65 M • 35 mL = X • 500 mL X = 0.82 M (rounded) You see that mL units canceled, leaving M unit, which is molarity, the concentration wanted. Note that C1 and C2 units must be the same and that V1 and V2 units must be the same when you use this formula. Try it his way: C1 = 11.65 mole/L (the same as 11.65 M) V1 = 0.035 L (the same as 35 mL) C2 = X V2 = 0.5 L (the same as 500 mL) Then 11.65 mole/L • 0.035 L = X • 0.5 L X = 0.82 mole/L (rounded)... same answer. You see that L canceled, leaving mole/L units. back to problem 6 Solution to problem 7: Since the drug is to be dispensed in milligrams per kilogram of body weight, first you need to convert the dog’s weight from pounds to kg. 21 lb. (body wt.) X 453.6 g X lb 1 kg = 1000 g 9.5 kg (rounded) Note the use of the proper system-to-system conversion factor (g/lb.), as required in the lab #1 instructions. The prescribed dosage is 2 mg of drug per kg of body weight. So, the total mass of the drug that the dog needs to receive is: 9.5 kg X 2 mg drug = kg 19.0 mg drug Where must this 19.0 mg come from? Answer: a bottle containing the drug at 1 g/100 mL. That is given. What volume of the bottled solution will provide 19.0 mg? 19 mg X __1g___ X 1000 mg back to problem 7 100 mL = 1g 1.9 mL is the volume to be dispensed from the bottle. Solution to problem 8: You're given a glucose solution at one concentration and asked to: a. prepare a solution of a different concentration, b. prepare exactly one liter of that new solution. One liter of the new solution will contain a total of 21,600 μg of glucose: 21.6 μg/mL X 1000 mL = 21,600 μg (of glucose). Note this is not a concentration expression. It is the total amount (mass) of glucose in the one liter of new solution asked for. Where are you to get that glucose? From the solution that you're given. So, how many milliliters of the given solution must you take in order to get 21,600 μg of glucose? 21,600 μg X _ 1 mg 1000 μg X _ 1 mL = 4.7 mg 4.6 mL 4.6 mL is a volume expression, which is the proper form for the answer to the question just asked. You see that μg and mg canceled. In other words, this says that 4.6 mL of the given glucose solution contains the 21,600 μg of glucose (the total mass) that you need. But recall (look above) where that 21,600 μg came from. That's the amount of glucose to be contained in one liter of new solution. So, to prepare the new solution you need to combine 4.6 ml of the given glucose solution with 995.4 mL of water. For best accuracy the 4.6 mL should be put into a 1 L volumetric flask after which water would be added to reach the 1 L mark on the neck of the flask. Alternate procedure: Since the total amount of solute taken from the given solution will be equal to the total amount of solute in the new solution that is prepared, we can use the C1V1 = C2V2 relation to solve this problem. Remember that the C1 and C2 units must be the same and that the V1 and V2 units must be the same. Given: C1 = 4700 μg/mL (derived from 4.7 mg/mL) V1 = X C2 = 21.6 μg/mL V2 = 1 L Then: 4700 μg/mL • X = 21.6 μg/mL • 1 L X = 0.0046 L. You see that μg/mL canceled. The answer here is in liter units. That’s equal to 4.6 mL...same answer as above. You could have converted V2 into mL and reached the same answer. OR you could have converted C2 into 0.0216 mg/mL and reached the same answer. Once more, remember that the C1 and C2 units must be the same and that the V1 and V2 units must be the same. AND recall that the C1V1 = C2V2 formula may be written as M1V1 = M2V2 where M1 and M2 are concentrations in molarity specifically. For the C1V1 = C2V2 formula, the concentrations may be expressed in ways other than molarity. back to problem 8 Solution to problem 9: How do you approach to problem? Convert everything to the same set of units, the same form of expression. Use g/L, for example. a. 1.5% (w/v) means 1.5 g solute per 100 mL of solution. 1.5 g_ X 100 mL 1000 mL = 1L 15 g L b. 15 μg X mL 1g X 106 μg 1000 mL = L 0.015 g mL c.. 150 mg X L d. 1.5 g L __1 g___ 1000 mg = 0.15 g L No conversion is necessary on this one. e. 0.15 mg X dL f. __1 g___ 1000 mg X 10 dL = L 1.5 X 10-3 g L 0.15 oz. X qt. 1.057 qt. X L 1 lb._ X 16 oz. 453.6 g = lb. 4.5 g L g. 1.5 X 10-3 oz. X gal. 1 gal. X 4 qt. 1.057 qt. X L 1 lb. X 16 oz. 453.6 g = lb. 0.011 g L So, which solution has the greatest solute concentration? (a) back to problem 9 Solution to problem 10: Consider how these two solutions would be prepared. If you dissolve 30 g of salt in water such that the final volume of the solution is 100 mL, then you have the first one. Remember that a solution is made of solute and solvent. Since the solute occupies physical space, some part of the space of that 100 mL of solution is taken up by the solute. Since you don't know exactly how much space is occupied by the solute, you don't know exactly how much water is in that 100 mL of solution, except that it is less than 100 mL of water. In preparing the other solution, you would add 30 g of salt to 100 mL of water and stir to dissolve the salt. Again, the salt takes up space. Since you started with 100 mL of water, the volume of the solution in this case must be somewhat greater than 100 mL. Thus, if you measured out 10 mL of each solution, there would be a lower concentration of salt in the second solution. back to problem 10 Solution to problem 11: “1:10 dilution” means to dilute the given solution by a factor of 10, which also means that the final solution’s concentration will be 1/10th that of the original. Intuitively you probably realize that you divide 1 M by 10 (or multiply 1 M by 0.1) to get 0.08 M as the concentration of the diluted solution. That’s true. But how do you prepare 30 mL of that? This is an example of a “parts” problem. For a 1:10 (= 1 in 10) dilution, divide the volume that is needed by 10. That gives 10 parts = 3 mL per part. Then combine 1 part (= 3 mL) of the given solution with 9 parts solvent (= 27 mL) to produce the new solution: 30 mL of 0.08 M alanine 0.8 M = 0.8 mole/L. 0.8 mole/L X 1 L/1000 mL X 3 mL = 2.4 X 10-3 mole. That amount (mass) of alanine in 3 mL will be combined with 27 mL of solvent. Therefore: 2.4 X 10-3 mole / 30 mL = 8 X 10-5 mole/mL. 8 X 10-5 mole/mL X 1000 mL/L = 0.08 mole/L = 0.08 M That’s the long way (stepwise) to calculate the concentration of the diluted solution. Shortcut: 0.8 M X 3 mL/30 mL = 0.08 M. Milliliters canceled. Note that 3 mL/30 mL is equivalent to 1/10 = 1:10 = 1 in 10. Suppose the problem was to prepare 55 mL of a 1:5 dilution of the 0.8 M alanine solution . Here the total number of “parts” in the new solution is 5. You would combine 1 part of the given solution (11 mL) with 4 parts of solvent (44 mL). 0.8 M X 11 mL/55 mL = 0.16 M Another example: Given a 2.1 mg/mL solution of glucose, prepare 80 mL of a 1:20 dilution. Concentration of diluted solution? One part of the new solution will be 80 mL/20 = 4 mL. Combine 4 mL of the 2.1 mg/mL solution with 76 mL solvent. New concentration: 2.1 mg/mL X 4 mL/80 mL = 0.105 mg/mL Another example: Given a 4.9 g/L solution of NaCl, prepare 100 mL of a 3:7 dilution. Concentration of diluted solution? Total number of parts of the new solution is 7. Therefore, 1 part of the new solution will be 100 mL/7 = 14.3 mL (rounded). 3 parts = 42.9 mL (rounded). Combine 42.9 mL of the 4.9 g/L NaCl solution with 57.1 mL of solvent (that’s 4 parts) to get the 100 mL of new solution. New concentration = 4.9 g/L X 3/7 = 2.1 g/L. That’s the same as 4.9 g/L X 42.9 mL57.1 mL. To check that answer: 4.9 g/L X 42.9 mL X 1 L/1000 mL = 0.21 g NaCl. Then 0.21 g/100mL = 0.21 g/0.1 L = 2.1 g/L (same answer) Another example: Given a 1 M NaCl solution. A lab procedure requires 50 mL of a 0.2 M NaCl solution. How do we prepare that? The previous problems show that the dilution factor is: concentration of diluted solution divided by concentration of original solution. Here, the dilution factor is 0.2 M / 1 M = 1/5 = 1:5 = 1 in 5. So, the 50 mL required has 5 parts, each equal to 10 mL. Combine 10 mL of 1 M NaCl with 40 mL of solvent to get 50 mL of 0.2 M NaCl. To check that answer: 1 M NaCl = 1 mole/L = 0.001 mole/mL. Then 0.001 mole/mL X 10 mL = 0.01 mole NaCl. 0.01 mole NaCl/50 mL = 0.0002 mole/mL = 0.2 mole/L = 0.2 M (same answer) You see that a few obvious steps (conversion factors) were skipped here? back to problem 11 Solution to problem 12: Question “a” From the previous parts dilution problems, we know that a 1:10 dilution of the original virus suspension will contain 5 X 104 virus particles. And a 1:10 dilution of that will contain 5 X 103 virus particles. And a 1:10 dilution of that will contain 5 X 102 virus particles. This is a series of dilutions, hence the name serial dilution. The dilution factors can be multiplied. So the second suspension prepared has a dilution factor of 1:100 relative to the original virus suspension. And the third suspension has a dilution factor of 1:1000 relative to the original suspension. Question “b” As to the preparation of the virus suspensions, the scientist is probably working with a small volume of the virus suspension material, since this is an infectious agent. Limiting the amount of infectious material in the experiment will limit the possibility of accidental exposure of lab personnel to the virus. Therefore, she won’t be preparing liter volumes of these virus suspensions. If she intends to treat a few-toseveral test animals with each dilution, then she might prepare 10 mL of each dilution, which would be enough to treat several animals with each dilution, at the rate of 1 mL per animal. Thus, 1 mL of the original virus suspension (5 X 105 virus particles per mL) plus 9 mL solvent will produce 10 mL of the suspension containing 5 X 104 virus particles per mL. Then 1 mL of this dilution plus 9 mL solvent will yield 10 mL of the suspension containing 5 X 103 virus particles. Then 1 mL of this dilution plus 9 mL solvent will yield 10 mL of the suspension containing 5 X 102 virus particles. So she ends up with 9 mL of the first diluted suspension, 9 mL of the second diluted suspension, and 10 mL of the third diluted suspension. She has her dilution series and the total amount of material being handled is small. Keeping the scale small, when possible, also reduces waste of materials. Summary: dilution factors in serial dilution problems can be multiplied, e.g.: 1/10 X 1/10 X 1/10 = 1/1000 total dilution 1/10 X 1/5 X 1/2 = 1/100 total dilution 1/3 X 1/3 X 1/3 = 1/27 total dilution 3/10 X 3/10 = 9/100 total dilution back to problem 12 A final note about written form in dilution problems: When a problem wants to dilute a solution by 1 to 10, that may be written as 1/10 or “by a factor of 10” or 1:10. This 1:10, like these other expressions that mean the same, translates as: “1 part of the given solution plus 9 parts of solvent;” i.e. 10 parts total for the solution being prepared. Somewhere you may see the expression written as 1:9 when the writer means “1 part of the given solution plus 9 parts of solvent.” Similarly 1:4 should mean “1 part of given solution plus 3 parts of solvent.” But it may be found as 1:3, meaning “1 part of given solution plus 3 parts of solvent.” These alternate expressions are incorrect and can cause confusion. How to remember it: the denominator of the dilution factor should be the total number of parts in the new solution to be prepared. 1:10 = 1/10 means 10 parts total. 1:5 = 1/5 means 5 parts total.