Multiple Choice Questions (2 points each) Which of the following is
advertisement
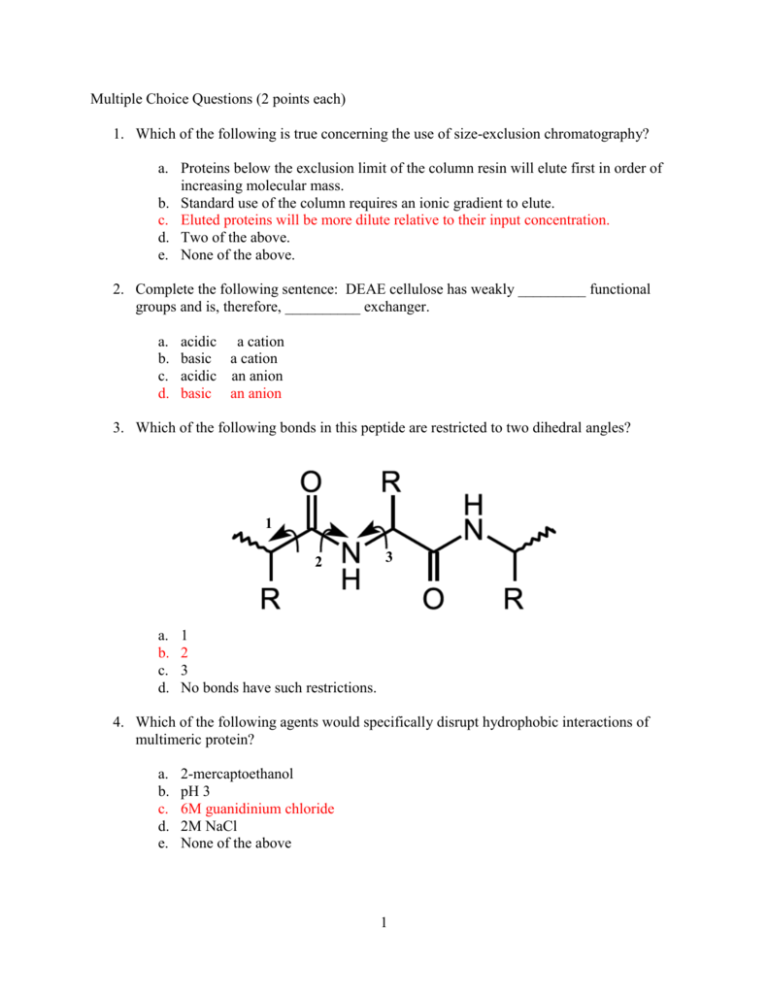
Multiple Choice Questions (2 points each) 1. Which of the following is true concerning the use of size-exclusion chromatography? a. Proteins below the exclusion limit of the column resin will elute first in order of increasing molecular mass. b. Standard use of the column requires an ionic gradient to elute. c. Eluted proteins will be more dilute relative to their input concentration. d. Two of the above. e. None of the above. 2. Complete the following sentence: DEAE cellulose has weakly _________ functional groups and is, therefore, __________ exchanger. a. b. c. d. acidic a cation basic a cation acidic an anion basic an anion 3. Which of the following bonds in this peptide are restricted to two dihedral angles? 1 2 a. b. c. d. 3 1 2 3 No bonds have such restrictions. 4. Which of the following agents would specifically disrupt hydrophobic interactions of multimeric protein? a. b. c. d. e. 2-mercaptoethanol pH 3 6M guanidinium chloride 2M NaCl None of the above 1 5. Myoglobin and the subunits of hemoglobin have a. b. c. d. e. no obvious structural relationship. very different primary and tertiary structures. very similar primary and tertiary structures. very similar primary structures, but different tertiary structures. very similar tertiary structures, but different primary structures. 6. Which of the following Levinthal “golf course” landscapes represent a purely random walk of an unfolded protein towards its native state N? a. c. b. d. 7. Which of the following is true concerning the increased stability of anti-parallel β-sheets over parallel β-sheets? a. Anti-parallel β-sheets are often found in the hydrophobic core of proteins. b. Backbone hydrogen-bonding atoms are parallel to each other in anti-parallel βsheets. c. The side chains in anti-parallel β-sheets have more stable packing conformation in anti-parallel β-sheets. d. Anti-parallel β-sheets are stabilized by adjacent salt bridges. e. They both have very similar stabilities although anti-parallel β-sheets are more prevalent. 2 8. Which of the following is true concerning insulin? a. b. c. d. e. Two genes encode for the A and B chains in insulin. Proteolytic cleavage occurs before disulfide bonds are formed. The protein is biologically active until proteolytic cleavage occurs. All of the above. None of the above. 9. Which statement is true concerning the structure of collagen? The individual helix components have more residues per turn than an α-helix. The superhelical structure is right-handed. The primary component residues are proline, hydroxyproline, and alanine. Collagen is strong due to side-chain hydrophobic interactions between adjacent helixes. e. None of the above. a. b. c. d. 10. Multiple choice, choose one or more correct choices. NMR spectroscopy is commonly used to measure the rate at which 1H is replaced by deuterium when a protein is dissolved in D2O; these deuterium exchange rates may provide information about: a. b. c. d. The molecular weight of a protein. The approximate radius of a protein in solution. How often hydrogen bonds open along a protein backbone. The approximate secondary structure content of a protein (such as % helix, % beta strand). e. Whether the deuterium nuclei are within about 6 Å of the 1H nuclei in the protein. f. The flexibility or rigidity of different parts of a protein structure. 3 Written Answer Questions 1. (10 points) (a.) Draw the structure of the tripeptide Arg-Glu-Thr, indicating the N- and C-terminii, the peptide bonds and the charges on the molecule at pH 1. (b.) Both parallel and anti-parallel forms of beta-pleated sheets are found in proteins. They differ in the pattern of hydrogen bonding between the chains and this, in turn, affects the stability of the overall beta-pleated sheets. Describe the differences in the H-bonding (an illustration plus text would be best). On the chart below, which shows a denaturation curve for a protein composed primarily of parallel-beta sheet structures, draw what the denaturation curve for a protein made up primarily antiparallel beta sheets would look like. 4 In parallel beta pleated sheets the O|| and NH groups on the two polypeptide C chains are not exactly opposite each other. As a result, the H-bonds between them are angled, which makes the bonds less stable than the interchain Hbonds in anti-parallel beta pleated sheets. As a result, anti-parallel beta pleated sheets are more stable than are parallel ones. (See plot on figure.) 2. (9 points) (a.) Water has an extremely high dielectric constant compared with other common solvents. Briefly describe how this high dielectric constant affects the solubility of an ionic compound such as sodium chloride, compared with a solvent such as acetone, which has a much lower dielectric constant. See page 42. The partial charges on water molecules cause them to be attracted to both positively charged ions, such as Na+ and negatively charged ions such as Cl- (see Figure 2-5). The high dielectric constant of these water molecules allows them to act as an effective insulator decreasing the tendency of the ions to coalesce, thereby increasing the solubility. (b.) The active sites of enzymes quite often have relatively few water molecules. Describe how the lowered dielectric constant of this region might affect the catalytic process. An active site with few water molecules would effectively have a lowered dielectric constant. This in turn would affect the pKs of amino acid side chains in the active site pocket (increasing them). This could allow, for example, a side chain carboxyl group to retain its H and act as a proton donor (acid), which it could not do in an aqueous environment. (c.) How does the partial double bond character of the peptide bond affect the ways in which a polypeptide chain can fold? The partial double bond character of the peptide bond greatly restricts the rigidity of the bond. Single bonds can rotate freely while double bonds cannot. Thus the Cα-C, C-0, N-H, and N-Cα groups of the peptide bonds are effectively in a plane and rotation is restricted mainly around the Cα-N and Cα-C bonds. Even here, steric considerations also restrict free rotation. 5 3. (9 points) Describe the general structure of collagen fibrils. What is unusual about the amino acid composition of collagen? What types of linkage help to stabilize mature collagen fibrils. Use diagrams as appropriate. See pages 234-239. The general structure of collagen fibrils is that they are arranged in a triple helical structure. Each of the strands is wound into a left-handed helix that is more extended than an alpha-helix. The three strands of the fibril are in turn wound around each other in a right-handed helix. The amino acid composition of collagens is unusual in that it has a very high percentage of glycine (about 30%) and also large amounts of proline and hydroxyproline. Mature collagens are stabilized by intra- and interchain covalent cross-links derived from lysine and histidine residues (see page 238). 4. (6 points) The major intracellular buffer is the acid/conjugate base pair H2PO4- / HPO42- with a pKa of 7.2. What pH is obtained from a solution prepared from a molar ration of Na2HPO4 / NaH2PO4 = 0.25. Show your work. Use the Henderson-Hasselbalch Equation pH = pK + log [ A- ] [HA] = 7.2 + log (0.25) = 7.2 – 0.6 = 6.6 6 5. (8 points) Deduce the amino acid sequence of the peptide from the following information. Show your work. (a.) Amino acid composition Lys, Met, Phe, Leu, Gly, Arg, Ala, Glu composition – only 1 residue of each amino acid is assumed (b.) Sanger’s dinitrofluorobenzene reagent, which reacts with amino groups, gives an alpha-amino derivative of alanine. Gives ala as N-terminus (c.) Trypsin treatment (cleaves on C-terminal side of Arg and Lys) gives three peptides with the following compositions: i) Glu, Arg, Ala ii) Phe, Lys, Met iii) Gly, Leu i) Peptide glu, ala, arg must be (ala, glu)-arg because arg at C-terminus. Because (b) gives ala as N-terminus, this peptide must be ala-glu-arg ii) Peptide (phe, lys, met) must have lys as C-terminus, therefore, peptide is (phe, met)-lys iii) Peptide (gly, leu) must be at C-terminus because it has no lys or arg. (d.) Cyanogen bromide (CNBr) treatment yields two peptides with the following composition. CNBr cleaves on the C-terminal side of methionine. i) Phe, Met, Glu, Arg, Ala ii) Leu, Gly, Lys iv) Peptide (leu, gly, lys) must be at C-terminus as it has no met. Lys must come before (leu, gly) from c) iii). Therefore, peptide is lys(leu,gly). Peptide (phe, met, glu, arg, ala) must be ala-glu-arg-phe-met because ala-glu-arg is N-terminal and met must be C-terminal. From the above information the overall peptide must be ala-glu-arg-phemet-lys (leu,gly). There is insufficient information order leu and lgy. 7 6. (9 points) You have cloned and expressed a human brain protein in E. Coli. You then purified this protein using, a variety of techniques. This resulted in a preparation that gave a single band of the expected molecular weight on SDS-gel electrophoresis. (a.) Briefly describe two procedures that would confirm that this purified protein is indeed the human protein you want. i) Edman degradation – could confirm N-terminal sequence and compare with known sequence from human genome. ii) Use an antibody that is known to cross-react with this protein acid and run an immunoassay (e.g., Western blot). iii) Do an amino acid composition analysis (very imprecise). iv) Use mass spectrometry to get a very exact molecular weight and compare this with expected molecular weight from gene sequence (b.) Name a procedure that could allow you to determine the native molecular weight of the protein. i) Native (non-denaturing) polyacrylamide gel ii) Gel filtration in non-denaturing solvent iii) Ultracentrifugation (c.) Note one of the ways in which the recombinant protein might differ from the protein as it exists in human brain cells. i) Lacks modified amino acids ii) Could lack covalently attached acetyl group iii) Could lack covalently attached sugars or lipids 8 (d.) In hemoglobin, the binding of oxygen to one of the four subunits causes a marked increase in the oxygen affinity of the other subunits. This results in an S-shaped or cooperative oxygen binding curve. Briefly explain why the binding of oxygen to myoglobin does not result in increased oxygen binding by other myoglobin chains. Binding of oxygen to one of the subunits of hemoglobin changes the shape of the subunit to which oxygen is bound. This in turn affects the intersubunit interactions resulting in changes in the conformation of the other subunits, which results in a higher affinity for oxygen of these subunits. For myoglobin, even though oxygen binding might cause a conformational change of the molecule, this change cannot be transmitted to other subunits of myoglobin because they are not in physical contact as myoglobin is monomeric. 7. (9 points) Consider the following interconversion, which occurs in glycolysis. Fructose 6phosphate glucose 6-phosphate; Keq = 1.97 (a.) What is G°’ for the reaction (assuming that the temperature is 25 °C)? G°’ = -RTlnKeq = -5.7log(1.97) = -1.7 KJ/mol (b.) If the concentration of fructose 6-phosphate is adjusted to 150 mM and that of glucose 6-phosphate is adjusted to 50 mM, what is G? G=G°’ + RTlnKeq = -1.7 KJ/mol + 5.7log(50/150) = -1.7KJ/mol – 2.7 KJ/mol = -4.4 KJ/mol (c.) Why are G°’ and G different? These values are different because G°’ is based on a fixed concentrations (1M) and temperature. G is a function of reactant and product concentrations, as well as temperature (but in biological systems we rarely consider temperature changes) 9 8. (7 points) Photosynthesis is arguably the most important metabolic pathway, since essentially all our food and fiber is made by the process. Photosynthetic “light reactions” harvest solar power and generate high energy chemicals like ATP and NADPH. In higher plants, photosynthesis moves electrons from H2O to NADP+. Given the half reactions: NADP+ + 2 e- + 2 H+ NADPH + H+ E0’ = -0.34 V ½ O2 + 2 e- + 2 H+ H2O E0’ = 0.84 V (a.) What is the voltage change for the reaction? Show your work. NADP+ + 2 e- + 2 H+ NADPH + H+ ½ O2 + 2 e- + 2 H+ H2O E0’ = -0.34 V -0.34 V E0’ = 0.84 V -0.84 V -1.18 V H2O + NADP+ + 2e- + 2H+ NADPH + H+ + ½ O2 + 2e- + 2H+ a) -1.18 V (b.) How much energy, in KJ/mole, is required to move 2 electrons from H2O to NADP+? Show your work. b) G˚ = -nFE˚ = -2(96.5 KJ/mol V)(-1.18 V) = 228 KJ/mol 9. (12 points) (a.) Aluminum-27 has a nuclear spin of 5/2. If a large number of aluminum-27 nuclei are placed in a magnet field, they will distribute themselves between _____six_________ allowed NMR energy states. (answer is a number) (b.) When a protein is dried from H2O solution and then dissolved in D2O under normal biochemical conditions of pH and temperature (such as pH 7, 25 degrees C), which protons on the glutamine and tyrosine residues will be replaced with deuterium? Draw the structures of glutamine and tyrosine as they would be in D2O, with appropriate H's replaced with D's. Draw Glu and Tyr as they would be in the context of a protein. Replace the hydrogens that are directly bonded to N or O with deuterium. 10 (c.) An important set of data in determining the structure of a protein or nucleic acid is a 2-dimensional nuclear Overhauser effect (NOE) spectrum, from which the intensity of the NOE between pairs of protons in a molecule can be quantitatively measured. What specific structural information does this spectrum provide? Distance between protons separated by approximately 6 Angstroms or less. (d.) As a protein structure determination method, what are two of the most significant advantages that NMR spectroscopy has relative to x-ray crystallography? Don't need crystals. Structure is determined in solution rather than solid state. Can obtain information regarding which parts of the protein are most flexible. There are probably some other advantages; you only need to state two. (e.) What is the term ENOE a measure of? In the context of simulated annealing, it is a measure of how much the inter-proton distance in the structural model differ from the distances derived from the experimental NOE data (or NMR data). 11