Lab Titration
advertisement

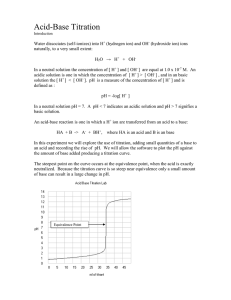
AP Chemistry LAB – Acid Base Titration Student ___________________ Date ______________________ History The titration process is used to determine the volume of a solution needed to react with a given amount of another compound. In this experiment we are going to titrate Hydrochloric Acid with a solution of Sodium Hydroxide. The concentration of Sodium Hydroxide will be given and you have to calculate the concentration of Hydrochloric Acid. Hydrogen ions from the HCl react with the hydroxide ions from Sodium Hydroxide to form water and salt in the overall reaction H Cl Na OH H 2O Cl When an acidic solution is titrated with a basic solution, the pH value for the acid will be initially low. As the base is added, the ph value begins to change gradually until close to the equivalent point, when equimolar amounts of acid and base have been mixed. Near the equivalent point the ph value begins to increase rapidly. The change in pH then becomes gradual again, before leveling off with the addition of excess base. In this experiment, we will use the ph meter to determine the pH values needed for graphing. The equivalence point and molarity of the acid solution will be determined. Pre-Lab Questions The questions are to be answered on another sheet of paper. Use complete sentences. 1. What is an “indicator”? 2. Write an example as to how titration is used to find the molarity of an unknown solution. 3. What is the “equivalent point?” 4. Can titration be used for determining the molarity of other solutions? Explain. Use an example in you explanation. 5. What always happens in an acid-base reaction? 6. What is a “volumetric analysis”? 7. What is a “stoichiometric point”? Materials needed Meter for determining pH 0.1 M solution of NaOH 50 ml Buret Buret assembly 10 ml pipette 250 ml beaker DI water Necessary clamps Safety Issues For this experiment, wear safety goggles at all times. You will be working with an acid and base, both of which can be harmful to your skin and especially your eyes. Procedure 1. 2. 3. 4. 5. 6. 7. 8. 9. Wear safety goggles. Use a pipette and add 10 ml of he HCI solution to a beaker. Add 50 ml of DI water to the beaker. Stir your solution for a time to ensure a complete mixture. Obtain a 50 ml buret and carefully add the .1 M solution of NaOH. Drain a small amount of the base solution to fill an empty portion of the buret. When you feel this experiment is set up, be sure to let the teacher examine your setup before you begin titration. Add 3 drops of Phenothalein Solution to the contents in the beaker. Begin titration by adding 1 ml amounts of the base, checking after addition of the pH of the solution with your meter. Set up a data sheet and record all data and observations. Processing Data 1. Be sure you have enough data to plot a true graph of your results that will adequately complete this experiment. You should see a well-represented graph that clearly defines the “equivalence POINT”. 2. Be sure to record any observations that you see that my cause errors in your data. 3. Be neat in the collection and final write-up for this experiment. 4. Show all calculations in a step-by-step manner. Data and Calculations Concentration of NaOH NaOH Volume added before largest pH increase NaOH Volume added after largest pH increase NaOH Volume added at Equivalence Point Moles of NaOH Moles of HCl Concentration of HCl Post Lab Questions Answer these questions on another sheet of paper. Be explicit in your answer. 1. What is this experiment trying to demonstrate to you as a student? 2. Check with other fellow students and compare your experimental results. Explain your error. Could an error analysis be done on this experiment? Why or why not? 3. How can titration be of benefit to anyone? 4. Check the Internet. How is titration being used in industry? 5. Write a paragraph that describes a conclusion for this experiment.