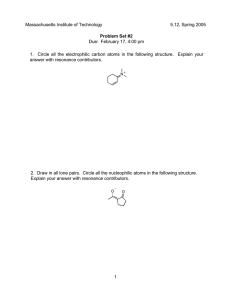
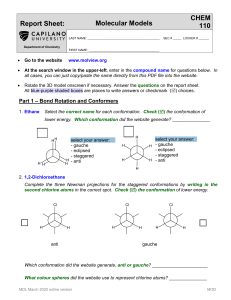
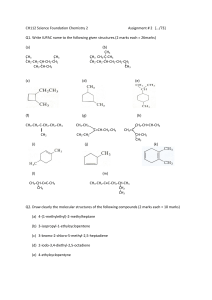
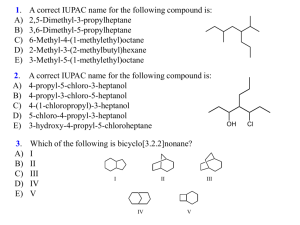
Organic Chemistry (1) Examination
advertisement

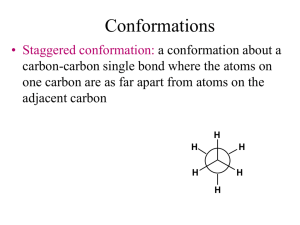
Organic Chemistry (1) Examination April 4, 2000 1.Give the IUPAC name for each of the following compounds. O (a) H3CH2CH2C C (c) C(CH3)3 H3CH2C CH3 (b) CH3 2.Write Lewis structure for each of the following compounds , and draw all possible resonance structure, and assign the formal charge and rank them in order of reactive important. (a) LiAlH 4 (b) HNO2 (d) CH3CO2- (c) CH2N2 3.Rank the following compounds in order of increasing acidity and explain the order. (a) CH3CH2OH, CH3CH2CO2H, ClCH 2CH2CO2H O O O (b) H C 3 C CH3, H3C C CH2 C CH3 4.Assign R or S to each stereocenter. (a) H (b) H3C OH (c) CH3 OH Cl HOH2C H H HOOC H C H CH2 5.Calculate the equilibrium constant, Keq, for the following acid – base reaction. The pKa of acetylene and ammonia are 25 and 38, respectively. HC Na NH 2 CH HC C Na NH3 6. Following is one of the enantiomers of 2–iodobutane. H H3CH2C I C CH3 (a) Is this (R)-2-iodobutane or (S)-2-iodobutane? (b) Draw a Fischer projection for this enantiomer. (c) Draw the Newman projections for all possible staggered conformations viewed along the bond between carbon 2 and 3 of this molecule. (d) Draw the enantiomer of this molecule. 7.Answer for each of the following questions about 2-isopropyl-5-methylcyclohexanol. (a) Draw structural formulas for all possible cis, trans isomers (b)Draw the more stable chair conformation for each of your answer in part(a). (c)Which is the most stable isomer? 8.Provide your explanation for each of the following observations. (a) The anti conformation of butane is more stable than the gauche conformation. (b) In chlorocyclohexane, the chair conformation having the chloro group in equatorial position is more stable than one having the chloro group in axial position. (c) Phenol, C6H5OH(pKa 9.95), is only slightly soluble in water, but its sodium salt, C 6H5O-Na+, is quite soluble in water. (d) 2,2-dimethylbutane(C6H14, bp 49.7℃) (e) Acetylene is more acidic than ethylene. (f) The most stable conformation of 1,4-di-tert-butylcyclohexane is not chair conformation. 9. Explain each of the followings. (a) torsional strain (b) inductive effect (c) dihedral angle (d) VSEPR model