Worksheet Chapter 4
advertisement
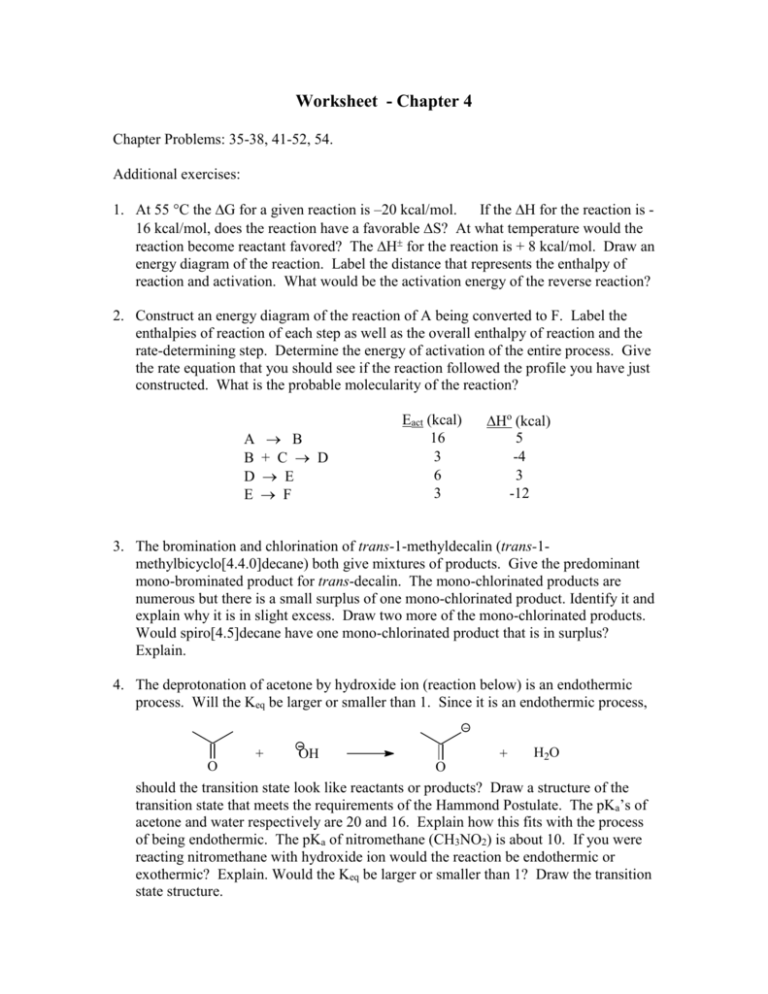
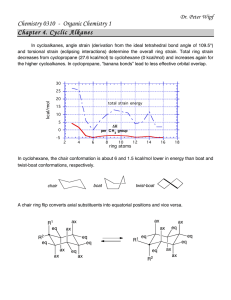
Worksheet - Chapter 4 Chapter Problems: 35-38, 41-52, 54. Additional exercises: 1. At 55 C the G for a given reaction is –20 kcal/mol. If the H for the reaction is 16 kcal/mol, does the reaction have a favorable S? At what temperature would the reaction become reactant favored? The H for the reaction is + 8 kcal/mol. Draw an energy diagram of the reaction. Label the distance that represents the enthalpy of reaction and activation. What would be the activation energy of the reverse reaction? 2. Construct an energy diagram of the reaction of A being converted to F. Label the enthalpies of reaction of each step as well as the overall enthalpy of reaction and the rate-determining step. Determine the energy of activation of the entire process. Give the rate equation that you should see if the reaction followed the profile you have just constructed. What is the probable molecularity of the reaction? A B D E B + C D E F Eact (kcal) 16 3 6 3 Ho (kcal) 5 -4 3 -12 3. The bromination and chlorination of trans-1-methyldecalin (trans-1methylbicyclo[4.4.0]decane) both give mixtures of products. Give the predominant mono-brominated product for trans-decalin. The mono-chlorinated products are numerous but there is a small surplus of one mono-chlorinated product. Identify it and explain why it is in slight excess. Draw two more of the mono-chlorinated products. Would spiro[4.5]decane have one mono-chlorinated product that is in surplus? Explain. 4. The deprotonation of acetone by hydroxide ion (reaction below) is an endothermic process. Will the Keq be larger or smaller than 1. Since it is an endothermic process, + O OH + O H2O should the transition state look like reactants or products? Draw a structure of the transition state that meets the requirements of the Hammond Postulate. The pKa’s of acetone and water respectively are 20 and 16. Explain how this fits with the process of being endothermic. The pKa of nitromethane (CH3NO2) is about 10. If you were reacting nitromethane with hydroxide ion would the reaction be endothermic or exothermic? Explain. Would the Keq be larger or smaller than 1? Draw the transition state structure.