SI Leader: Sullivan Hanback Name Course: General Chemistry 115
advertisement

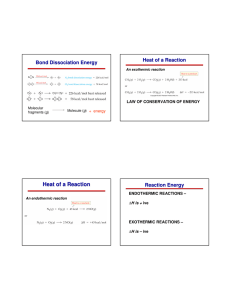
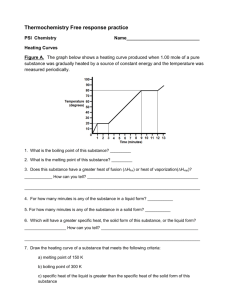
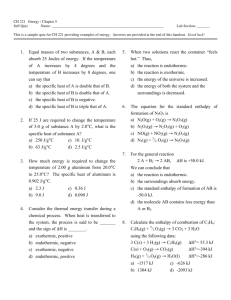
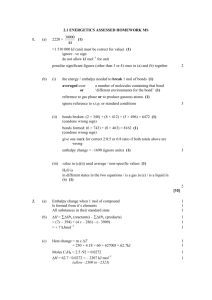
SI Leader: Sullivan Hanback Name ________________________ Course: General Chemistry 115 2B Worksheet 21 1. What is the difference between potential and kinetic energy? 2. Indicate whether each of the following possesses potential or kinetic energy. a. Diver on a diving board b. Cheetah chasing a gazelle c. Electron attracted to a nucleus d. Energy stored in an C-O bond e. Atoms bouncing around in a solution 3. How does endothermic and exothermic processes relate to heat and work? 4. What is heat capacity? What is the difference between molar heat capacity and specific heat capacity? 5. What is the specific heat of gold if when heating 0.025kg of the metal the temperature changes from 20°C to 74°C when 5,200J of heat is applied? 6. For each of the following indicate the appropriate phase transitions and whether each process is endothermic or exothermic. a. Enthalpy of fusion b. Enthalpy of vaporization c. Enthalpy of condensation d. Enthalpy of freezing 7. 4456J of heat is used to vaporize a glass of water. How many grams of water are in the glass? (∆HCondensation = -2260J/g) 8. Calculate the total amount of heat required to convert 950g of ice at -45°C to liquid water at 69°C. Given: the specific heat of ice is 2.03 J/g°C, specific heat of water is 4.184 J/g°C, and enthalpy of fusion is 333 J/g. 9. Given the reaction below. How much heat is released when 68 g of oxygen is reacted with excess C4H10.? C4H10 + O2 CO2 + H2O ∆H= -2856kJ 10. Calculate the heat of reaction for the following reaction given the reaction below. CH4 (g) + Cl2 (g) CH3Cl (g) + HCl (g) O==O 502 kJ/mol C==O 730 kJ/mol O—H 464 kJ/mol H—H 435 kJ/mol Cl—Cl 243 kJ/mol H—Cl 431 kJ/mol C—Cl 331 kJ/mol C—H 414 kJ/mol