Regents level kinetics and equilibrium review
advertisement

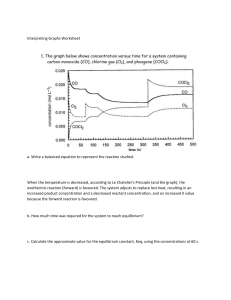
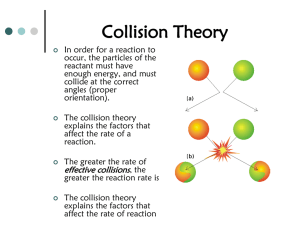
Regents level kinetics and equilibrium review 1. all reactions need collisions to take place, the more frequent the collisions the faster the reactions and the less time needed 2. Increased temperature, increased concentration(molarity), increased surface area(powder), and a catalyst all make reactions faster 3. Catalyst provide an alternate pathway with lower activation energy 4. physical and chemical systems may go to equilibrium in a closed system 5. saturated solutions are at equilibrium, rate of dissolving = rate of crystallization 6. freezing point, boiling point, closed bottle with water in it all have equilibrium 7. RECC Rates Equal Concentrations Constant 8. increasing a reactant concentration shifts a system to the right which increases product and decreases the reactants – you can’t make product without consuming reactant 9. decreasing a concentration shifts to the same side you decreased from 10. adding heat favors the endothermic process – remember ice melts better with more heat 11. increasing pressure will shift to the side with less moles of gas, if moles of gas are not different pressure has no effect 12. a catalyst does not cause a shift, it only make the system reach equilibrium faster K > 1 products are favored K< 1 reactants are favored K = [products]x [Reactants] y each is raised to the power of the coefficients Solids and liquids are left out