Lab 1-2 Penny Density Lab
advertisement

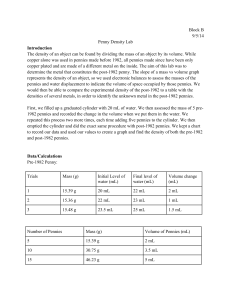
Chemistry I Lab 1-2 Penny Density Lab In this lab, you will graphically determine and compare the density of two sets of pennies. Penny Set A was minted before 1982. Penny Set B was minted after 1982. In 1982, the U.S. Mint changed the composition of pennies. Before 1982, pennies were made entirely of copper. After 1982, pennies were made of zinc with a copper coating. Think about what you learned about the densities of copper and zinc earlier in the unit. Pre-Lab Questions: 1. How are the pre-1982 and post-1982 pennies different from each other? 2. How is the density of copper different from the density of zinc? 3. It is important for the pennies to be dry before the mass or volume is determined. Suppose you found the mass of wet pennies. Would the density be too high or too low? 4. When you drop the pennies in the graduated cylinder, suppose some water splashes out. Would the volume measurement of the pennies be too high or too low? Would the density be too high or too low? Hypothesis Write a hypothesis based on the purpose of the lab. Include an explanation for your prediction. Data Table Prepare a data table to collect the information. What measurements do you need to make to find the density? Put a title at the top of the table and label columns for those for each set. Data Analysis/Conclusion: 1. Create a graph of your data. Attach your graph to your lab report. You will have two lines on your graph (A—Pre-1982 and B—Post-1982). Your graph should have: descriptive title labeled axes w/units best-fit lines legend (key) to distinguish both lines equations of the lines R2 values 2. Use the information from your graph to complete the table for each line. Data Set Equation of Best-Fit Line Slope of Line (w/units) R2 A Pre-1982 B Post-1982 3. a. What is the density of the A (pre-82) pennies? of the B (post-82) pennies? b. Which set of pennies (Pre-1982 or Post-1982) is more dense? c. Was your hypothesis supported or rejected by the data? d. Based on what you know about the density of zinc and copper, explain the difference in the density of the two sets of pennies. 4. Discuss the precision of your data. List one possible experimental error that could impact the precision of your data. Why is graphing a preferred method of determining density instead of calculating it based on one mass and volume measurement? 5. How would the density of 10 pre-1982 pennies compare to the density of 20 pre-1982 pennies? Explain your answer. 6. If you had a penny and the date was unreadable, how could you determine if it was minted before or after 1982? 7. Think of another example in the world where determining the density of a substance would be useful in identification. Describe the situation and why density would be important in the identification. (Points for creativity. You will not get full points if you copy another student's answer).