Penny Isotopes - mrmcknightschem.info
advertisement

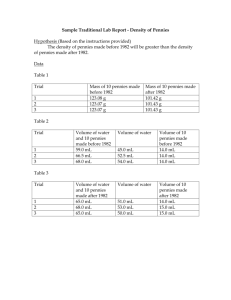
Penny Isotopes You will need some paper and a calculator for this lab. 1. Mass of pennies • Record the number of pennies in your container. List them by year – then record the mass of each penny next to the year it was manufactured. Use the electronic balances and be sure to record ALL digits on the display. Some years may be duplicated – record each penny individually. 2. Pennies as an analogy for Isotopes(?) • Pennies manufactured prior to 1982 were 95% copper, 5% zinc. • Pennies manufactured after 1982 are 5% copper, 95% zinc. • Pennies manufactured in 1982 are a mix of the older and newer types. • So pennies seem to have 2 ‘isotopes.’ 3. Averages • Calculate the average mass of the pennies in your sample which were made before 1982. • Calculate the average mass of the pennies in your sample which were made after 1982. 4. Average Atomic Mass • Use the formula: Average Atomic Mass = (% of 1st Isotope X Mass of 1st Isotope) + (% of 2nd Isotope X Mass of 2nd Isotope) Calculate: % of pre-1982 pennies in your sample. % of post-1982 pennies in your sample. 4. Continued • Use the average masses you calculated in Step 3 and the % of each isotope to calculate the ‘Average Atomic Mass’ of a penny. (Remember to either change % to a decimal – 50% is .5, OR divide your final answer by 100. I prefer to change % to a decimal at the beginning, since it leaves one less thing to forget.) 5. Density • Based on the average masses for pre- and post-1982 pennies and the composition information given in slide 2, which metal must be more dense – Copper or Zinc? • Use your textbook, the internet, or some other source to verify your answer. Record the actual densities of copper and zinc here. 6. Unknown Sample Mass of Unknown Sample: # of Pennies In Unknown Sample: Average Mass of Pennies in Unknown Sample: 6. Continued • Average Mass = (% 1 X Mass 1) + (% 2 X Mass 2) + …. • • • • If there are ONLY 2 ISOTOPES, Then (%1) + (%2) = 1 = 100% So: (%2) = 1 – (% 1) Substitute (1 - % 1) in place of (% 2) in the top equation. 6. Continued • Now solve for (% 1.) • 1 – (%1) = (%2) • In the unknown sample: • What is the percent of pre-1982 pennies? • What is the number of pre-1982 pennies? 7. 1982 • A sample of ____ pennies minted in 1982 has a mass of ________ grams. • If this sample is representative of all pennies minted in 1982 (this is doubtful) then what percent of pennies minted in 1982 were 95% copper? 8. Error • A student calculated the ‘average atomic mass’ of their sample of pennies to be 255.92 grams. • Is this a reasonable answer? • If not, what was the student’s most likely mistake?