HW8.docx
advertisement

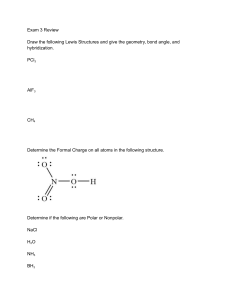
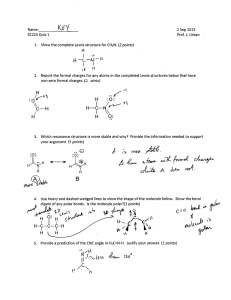
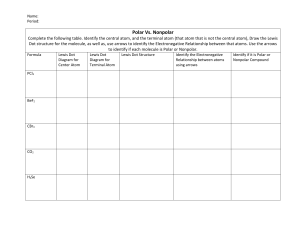
Name ____________________ CHEM 1004 Homework #8 Spring 2011 Buckley This homework is due on Tuesday, March 1, at class time. The assignment will be accepted until the start of class on Wednesday, March 2, with a 20% penalty. Assignments turned in after class that day will receive no credit, though I will look through them if you want me to. 1. (10 points) Write formulas for and name compounds formed between the following species. Formula Name a. Mg and S ___________________ ________________________ b. Cs and N ___________________ ________________________ c. Ba and PO43- ___________________ ________________________ d. Zn and Br ___________________ ________________________ e. Fe3+ and SO42- ___________________ ________________________ 2. (5 points) Write the formula for the following compounds. Formula a. calcium hydroxide ______________________ b. ammonium sulfide ______________________ c. lead (IV) nitrate ______________________ d. gold (III) nitride ______________________ e. diphosphorous pentoxide ______________________ ←←←←←PLEASE TURN THE PAGE OVER→→→→→ 3. (20 points) For the following species: a. Draw the Lewis structure. b. State the molecular shape. c. State whether the molecule is polar or nonpolar. Species CHF3 NH4+ SO3 BrO3- N2H4 Lewis Structure Molecular Shape Polar or Nonpolar?