Determining the Ka of Acetic Acid - SCH4U1-02-2010
advertisement
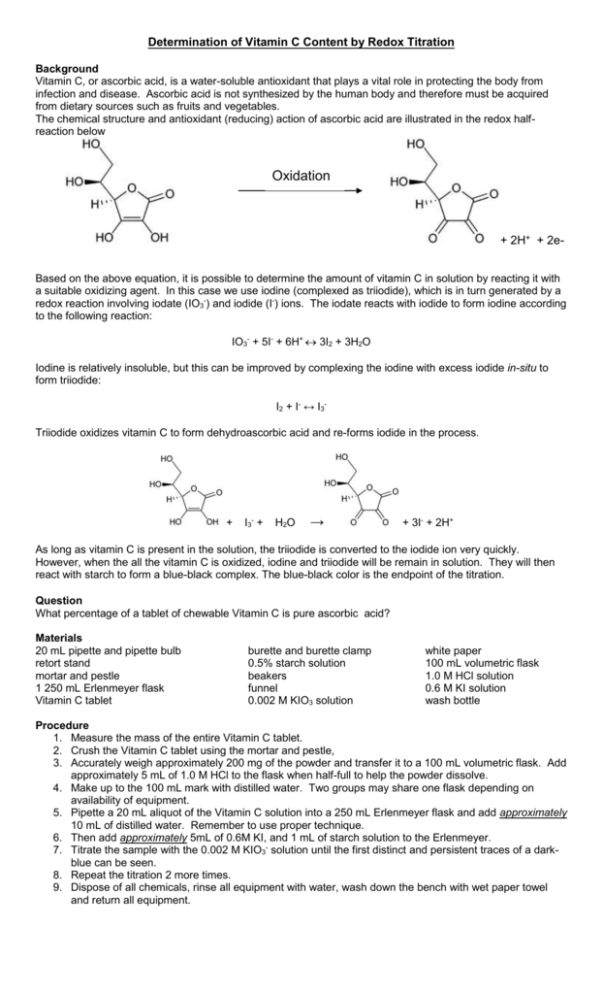
Determination of Vitamin C Content by Redox Titration Background Vitamin C, or ascorbic acid, is a water-soluble antioxidant that plays a vital role in protecting the body from infection and disease. Ascorbic acid is not synthesized by the human body and therefore must be acquired from dietary sources such as fruits and vegetables. The chemical structure and antioxidant (reducing) action of ascorbic acid are illustrated in the redox halfreaction below Oxidation + 2H+ + 2eBased on the above equation, it is possible to determine the amount of vitamin C in solution by reacting it with a suitable oxidizing agent. In this case we use iodine (complexed as triiodide), which is in turn generated by a redox reaction involving iodate (IO3-) and iodide (I-) ions. The iodate reacts with iodide to form iodine according to the following reaction: IO3- + 5I- + 6H+ 3I2 + 3H2O Iodine is relatively insoluble, but this can be improved by complexing the iodine with excess iodide in-situ to form triiodide: I2 + I- ↔ I3Triiodide oxidizes vitamin C to form dehydroascorbic acid and re-forms iodide in the process. + I3- + H2O → + 3I- + 2H+ As long as vitamin C is present in the solution, the triiodide is converted to the iodide ion very quickly. However, when the all the vitamin C is oxidized, iodine and triiodide will be remain in solution. They will then react with starch to form a blue-black complex. The blue-black color is the endpoint of the titration. Question What percentage of a tablet of chewable Vitamin C is pure ascorbic acid? Materials 20 mL pipette and pipette bulb retort stand mortar and pestle 1 250 mL Erlenmeyer flask Vitamin C tablet burette and burette clamp 0.5% starch solution beakers funnel 0.002 M KIO3 solution white paper 100 mL volumetric flask 1.0 M HCl solution 0.6 M KI solution wash bottle Procedure 1. Measure the mass of the entire Vitamin C tablet. 2. Crush the Vitamin C tablet using the mortar and pestle, 3. Accurately weigh approximately 200 mg of the powder and transfer it to a 100 mL volumetric flask. Add approximately 5 mL of 1.0 M HCl to the flask when half-full to help the powder dissolve. 4. Make up to the 100 mL mark with distilled water. Two groups may share one flask depending on availability of equipment. 5. Pipette a 20 mL aliquot of the Vitamin C solution into a 250 mL Erlenmeyer flask and add approximately 10 mL of distilled water. Remember to use proper technique. 6. Then add approximately 5mL of 0.6M KI, and 1 mL of starch solution to the Erlenmeyer. 7. Titrate the sample with the 0.002 M KIO3- solution until the first distinct and persistent traces of a darkblue can be seen. 8. Repeat the titration 2 more times. 9. Dispose of all chemicals, rinse all equipment with water, wash down the bench with wet paper towel and return all equipment. Analysis 1. Write the balanced half reactions between iodate and iodide, and between ascorbic acid and the liberated iodine. 2. Identify the atoms that are being oxidized and reduced in the reaction and label the oxidation numbers for these atoms. 3. Calculate the average volume of iodate solution used. 4. Calculate the number of moles of iodate reacting and the number of moles of iodine liberated. 5. Calculate the number of moles of ascorbic acid oxidized in the Erlenmeyer flask. Throughout all of these calculations, take care to mind the correct mole to mole ratios. 6. Calculate the concentration of the ascorbic acid in the volumetric flask in mol/L. 7. Calculate the number of milligrams of Vitamin C in the original tablet. 8. What percentage of the tablet is in fact Vitamin C? 9. Is the number reasonable? What are some explanations for any percentage error?









