Thermochemistry—Chapters 6, 9
advertisement

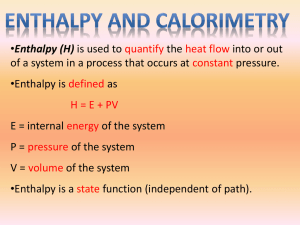
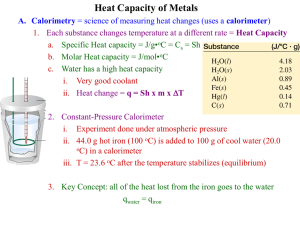
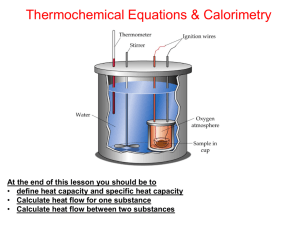
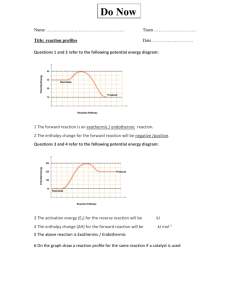
Chapter Six—Principles of Reactivity: Energy and Chemical Reactions VOCABULARY--Define and review the following terms prior to our lecture: Thermodynamics Energy Potential Energy First Law of Thermodynamics Heat Temperature System Surroundings Endothermic Exothermic Heat Capacity Specific Heat Capacity Internal Energy Work Enthalpy State Function Calorimetry: E = q + w w = - P V E = internal energy H = E at constant V E = q - PV q = H at constant P Temperature Units: Kelvin (K) is the SI unit for temperature o K = 273.15 + oC C = (5/9) (oF – 32) Heat Units: Joule (J) is the SI unit for thermal energy. 1 calorie = 4.184 J 1 J = 1 kg m2 / s2 1 Cal = 1000 cal = 1 kcal 1000 J = 1 kJ Example 1: How much energy is needed to heat a 10.0 g piece of copper metal from 25oC to 325oC? The specific heat of copper is 0.385 J/goC. Example 2: A 55.0 g piece of metal is heated in boiling water to 99.8oC and then dropped into a calorimeter of 225 mL of water at 21.0oC. The final temperature of the water and metal is 23.1oC. What is the specific heat of the metal? Example 3: You mix 200. mL of 0.400 M HCl with 200. mL of 0.400 M NaOH in a coffee-cup calorimeter. The temperature of the solutions before mixing was 25.10oC; after mixing the temperature is 27.78oC. What is the molar enthalpy of neutralization of the acid? (let the densities of all solutions be 1.00 g/mL and their specific heat capacities be 4.20 J/goC) Example 4: A 1.00 g sample of sucrose (C12H22O11) is burned in a bomb calorimeter. The temperature of the 1500. g of water in the calorimeter rises from 25.00oC to 27.32oC. The heat capacity of the bomb is 837 J/K. a) calculate the heat evolved per gram of sucrose. b) calculate the heat of combustion for sucrose. HEATING CURVES: Changes of State (Phase Changes) vaporization condensation sublimation deposition solidification fusion Example 5: How much heat is needed to convert 500.0 g of ice at –25oC to steam at 200oC? Specific heat of ice = 2.06 J/g K Specific heat of water = 4.184 J/g K Specific heat of steam = 1.92 J/g K Heat of fusion of ice = 333 J/g Heat of vaporization of water = 2256 J/g Energy Diagrams: H rxn = ENDOTHERMIC H = EXOTHERMIC H = Standard Enthalpy of Formation: Standard State: Example 6: a) Using standard heats of formation, calculate the molar enthalpy change for the combustion of methane, CH4. b) How much energy would be released if you combusted 200.0 g of CH4? Example 7: What quantity of energy must be supplied to sublime 10.0 g I2? I2 (s) I2 (g) H = 62.4 kJ Hess’ Law of Heat Summation: If a reaction can be written as the _____ of 2 or more other reactions, the ____ for the overall reaction is the sum of the _____ of those other reactions. Example 8: Use the following to calculate the standard heat of formation of methane: C (s) + O2 (g) CO2 (g) H = -393.5 kJ H2 (g) + ½ O2 (g) H2O (l) H = -285.8 kJ CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (l) H = -890.3 kJ Example 9: Calculate the standard heat of formation of B2H6 (g): 4B (s) + 3O2 (g) 2B2O3 (s) H = -2543.8 kJ 2H2 (g) + O2 (g) 2H2O (g) H = -484 kJ B2H6 (g) + 3O2 (g) B2O3 (s) + 3H2O (g) H = -2032.9 kJ HW Chapter 6: 14,22,26,30,32,34,38,42,44,60,74,92