Chemistry Notes – Chapter 4 – Atomic Structure
advertisement

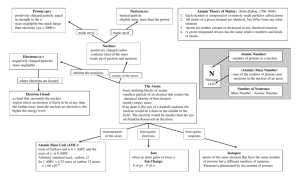
Chapter 3 –Chemical Foundations: Elements, Atoms, and Ions The Elements The early Greeks thought the universe was made of only four elements: air, earth, fire and water. Today we know of over 100 different elements, from which millions of known substances are composed of. Just like the alphabet, with its 26 letters, can be used to construct many different words, the elements of the periodic table can be used to construct an almost infinite number of compounds. The earth is made of oxygen (49%), silicon (26%), aluminum (7.5 %), and a host of other elements. The human body is also made of a lot of oxygen (65%), but in addition has a lot of carbon (18%) and hydrogen (10%). There are also a lot of trace elements in the body (trace elements are needed for life, but are present in very small amounts) such as arsenic, chromium and cobalt. The names from the elements come from many different sources, such as the Latin or Greek word for the element, or the place where it was discovered. Some elements are even named after a person, like Einsteinium. The symbols for each element are therefore a little curious, especially since many don’t begin with the first letter of the element (for example, W is the symbol for tungsten). To write an element symbol, the first letter is always capitalized and the second letter (if there is one) is lowercase. Dalton’s Atomic Theory John Dalton (1766-1844) was a school teacher who also conducted scientific experiments on the side. In the early 1800’s, some basic ideas about matter were generally accepted by scientists: Most natural materials are mixtures of pure substances. Pure substances are either elements or combinations of elements. A given compound always contains the same proportions by mass of the elements. John Dalton took these ideas and formalized them into his now-famous Dalton‘s Atomic Theory: 1) All elements are composed of tiny particles called atoms. 2) Atoms of the same element are identical. 3) The atoms of a given element are different from those of any other element. 4) Atoms of one element can combine with atoms of other elements to form compounds. 5) Atoms are indivisible in chemical processes. A chemical reaction simply changes the way the atoms are grouped together. Subatomic Particles particle symbol charge mass (amu)* location electron proton neutron ep+ no -1 +1 0 1/1840 1 1 e- cloud nucleus nucleus *1 amu = 1.66 X 10-24 g The first subatomic particle discovered was the electron. In the late 1890’s, J.J. Thomson set up a cathode ray tube and hooked it up to a battery. The resulting stream of electrons excited the gas particles in the tube and caused it to glow. EX: Draw a schematic of a cathode ray tube below: It was discovered by Ernest Rutherford that the protons and neutrons were located in a very small region called the nucleus. This experiment was done by shooting some positively charged particles at some gold foil. EX: Draw a schematic of Rutherford’s famous gold foil experiement. Atomic Number The atomic number (sometimes given the symbol “Z”) is the number of protons in the nucleus. This number is what gives an element its identity. For example, any atom with 6 protons in its nucleus is carbon. The periodic table is arranged in order of increasing atomic number. Mass Number Atoms of the same element can differ in the number of neutrons in the nucleus. Such variations lead to isotopes, which have the same number of protons, but different numbers of neutrons. The mass number of an atom is the number of protons + the number of neutrons the atom has. EX: What is the mass number of a carbon atom that has 5 neutrons in its nucleus? EX: How many nuetrons will a calcium atom have if its mass number is 42? To symbolize an isotope, we can use the following notation: mass number mass number atomic number atomic number The atomic number is redundant information, and is sometimes left off. For example, the “2” shown above for helium is unnecessary, since all helium atoms have two protons. We name an isotope by writing the element name followed by a dash and then the mass number. The above examples would be called helium-4 and helium-5, respectively. The only exception to this would be hydrogen. Hydrogen-1 is sometimes called just “hydrogen”. Hydrogen-2 is sometimes called “deuterium” and hydrogen-3 is sometimes called “tritium”. (Most hydrogen found in nature is hydrogen-1.) EX: Write the symbol and determine the number of neutrons for a gold-197 atom. FYI- an “amu” is 1/12 the mass of a carbon-12 atom. Amu stands for atomic mass unit. Atomic Mass (not in book) Each isotope of each element exists in nature in different abundances. The atomic mass is the weighted average of all the isotopes of that element. For example, about 99% of all carbon atoms are carbon-12. About 1% are carbon-13. Thus, the weighted average is somewhere between 12 and 13 amu. Since carbon-12 is much more abundant, the average is shifted closer to 12 than to 13. A periodic table shows that carbon has an atomic mass of 12.011 amu. EX: What would you guess is the most common isotope for Scandium? What would its symbol be, and how many protons, neutrons, and electrons would it have? Calculating Atomic Mass EX: The element copper is found to contain the naturally occurring isotopes Cu-63 and Cu-65. Their abundance is 69.17% and 30.83%, respectively, and their masses are 62.9296 amu and 64.9278 amu. Calculate the atomic mass for copper. EX: Neon has three isotopes with the % abundance and mass given for each. Use this information to calculate the atomic mass of neon. isotope Neon-20 Neon-21 Neon-22 mass (in amu) 19.992 20.994 21.991 abundance 90.48 % 0.27 % 9.25 % Electrons and Atomic Structure We will re-visit electrons and their role in the chemical and physical properties of elements later on. Here, we give just a brief introduction to the electron and it’s role in ion formation and flame tests. Formation of Ions A neutral atom has no net charge. The electrons and protons are in equal numbers, so the + and – charges cancel out. An atom can form an ion, however, when it gains or loses electrons. An atom that loses e- is called a cation and has a positive charge. An atom that gains e- is called an anion, and has a negative charge. The metals typically lose electrons to form cations and the nonmetals usually gain electrons to form anions. EX: A typical potassium ion (K+) has how many e-, no, and p+? EX: A typical oxide ion (O2-) has how many e-, no, and p+? The Bohr Model of the Atom and Flame Tests In 1913 Niels Bohr proposed a model in which the electrons circled the nucleus, like the planets orbit the sun. This model is sometimes called the planetary model. This model also proposed a very insightful idea: that the electrons could only occupy certain positions around the nucleus, and the farther out electrons got, the greater the electron’s energy. An electron could only move to a higher (or lower) energy level if it acquired (or lost) a certain amount of energy. This idea explains the phenomena observed in flame tests Mass Defect (not in book) You may notice that some atoms seem particularly light. In the example above, the mass of each isotope of neon is slightly less than you would predict. For example, Neon-20, with 10 protons,10 neutrons and 10 electrons has a mass of 19.992 amu (you would expect it to have a mass of just over 20 amu). This slight mass deficiency is due to the fact that some of the mass of the atom is converted to energy (called nuclear binding energy) to hold the nucleus together. The amount of mass lost can be used to calculate the nuclear binding energy by using Einstein’s famous equation E = mc2. EX: A proton has a mass of 1.00728 amu, while a neutron has a mass of 1.00867 amu. The electron has a very small mass of 0.00055 amu. Given this information, calculate the mass defect (the “missing mass”) of an oxygen-18 atom, which has a mass of 17.99916 amu.