SI Comment 16
BI-WEEKLY COMMENTS ON SI
SI Comment 16
THE MOLE
(
PART
2)
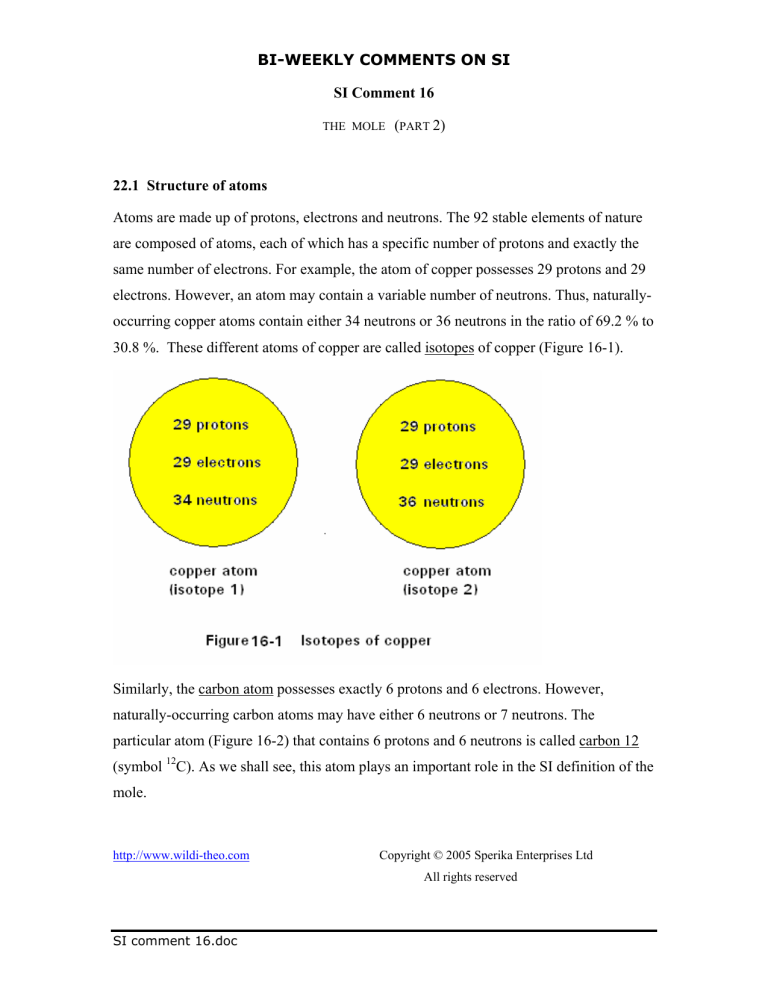
22.1 Structure of atoms
Atoms are made up of protons, electrons and neutrons. The 92 stable elements of nature are composed of atoms, each of which has a specific number of protons and exactly the same number of electrons. For example, the atom of copper possesses 29 protons and 29 electrons. However, an atom may contain a variable number of neutrons. Thus, naturallyoccurring copper atoms contain either 34 neutrons or 36 neutrons in the ratio of 69.2 % to
30.8 %. These different atoms of copper are called isotopes of copper (Figure 16-1).
Similarly, the carbon atom possesses exactly 6 protons and 6 electrons. However, naturally-occurring carbon atoms may have either 6 neutrons or 7 neutrons. The particular atom (Figure 16-2) that contains 6 protons and 6 neutrons is called carbon 12
(symbol
12
C). As we shall see, this atom plays an important role in the SI definition of the mole. http://www.wildi-theo.com
Copyright © 2005 Sperika Enterprises Ltd
All rights reserved
SI comment 16.doc
.
16.2 Atomic mass
Scientists have succeeded in determining the relative masses of the 103 atoms that occur naturally in the world. In doing so, they assigned the relative value of 12 to the mass of the carbon 12 atom. The carbon 12 atom is therefore said to possess an atomic mass of
12. The atomic masses of all the other naturally-occurring atoms are based on this number. For example, the naturally-occurring copper atom has an atomic mass of 63.55, where 63.55 is a pure number. A copper atom is therefore 63.55/12 = 5.29 times heavier than a carbon 12 atom.
Table 16.1 shows the atomic masses of 10 common elements, along with some of their other properties.
16.3 Molar mass
The mass of any atom is so small that it cannot be measured directly. However, scientists have assigned the mass of 12 grams (0.012 kg) to one mole of the carbon 12 atom. This important definition of the base value of molar mass enables us to determine the molar mass of any other atom, and indeed of any molecule whose atomic composition is known.
Thus, the mole is not merely a special number (6.022 × 10
23
), but it can now be used to determine the mass of any chemical compound. http://www.wildi-theo.com
Copyright © 2005 Sperika Enterprises Ltd
All rights reserved
SI comment 16.doc
Table 16.1 shows that the molar mass (grams per mole) is numerically equal to the atomic mass. The following examples illustrate the use that can be made of the molar mass.
TABLE 16-1
(pure number) g/mol hydrogen H 1 1 0, 1.008 1.008 carbon C 6 6 6, 12.01 12.01 carbon 12
12
C 6 6 6 12.00 12.00 nitrogen N 7 7 7, 14.01 14.01 oxygen O 8 8 8,9,10 16.00 16.00 aluminum Al 13 13 14 26.98 26.98 copper Cu 29 29 34,36 63.55 63.55 silver Ag 47 47 60, 107.9 gold Au 79 79 118 197.0 197.0
200.6 200.6
124
16.4 Manipulating moles and masses
Example 1
How many moles are there in 2.3 kg of copper?
Referring to Table 16.1, we note that the molar mass of copper is 63.55 g/mol. Since
2.3 kg = 2300 g, the number of moles of copper is: 2300 g/(63.55 g/mol) = 36.19 mol.
The number of copper atoms in this 2.3 kg mass is:
36.19 × 6.022 × 10
23
atoms = 2.18 × 10
25
atoms of copper.
Example 2
Water molecules (H
2
O) are composed of 2 atoms of hydrogen (H) and one atom of oxygen (O). What is the mass of one mole of water?
SI comment 16.doc
One mole of water is composed of 2 moles of hydrogen atoms and one mole of oxygen atoms. The mass of one mole of water is therefore:
2 mol (hydrogen atom) × 1.008 g/mol + 1 mol (oxygen atom) × 16.00 g/mol = 18.016 g.
The molar mass of water is therefore 18.016 g/mol.
Example 3
Oxygen, as a gas, is composed of oxygen molecules (O
2
), each of which is made up of
2 atoms of oxygen. How many moles of oxygen molecules are there in one pound of oxygen gas?
Using the Wildi SI Chart,
⊗
MASS , 1 lb = 453.59 g. Referring to Table 16.1, one atom of oxygen has an atomic mass of 16.00. Consequently, one molecule of oxugen has an atomic mass of 16.00 × 2 = 32.00. The molar mass of oxygen gas is therefore 32 g/mol.
Consequently, one pound of oxygen gas is equivalent to 453.59 g/(32.00 g/mol)
= 14.17 mol.
16.5 Just for fun – try this SI quiz
[1] The carbon monoxide gas molecule is composed of one atom of carbon and one atom of oxygen. What is the molar mass of the CO molecule?
[2] The propane molecule (C
3
H
8
) is composed of 3 atoms of carbon and 8 atoms of hydrogen.
How many moles are there in 260 grams of propane?
The solutions to this SI quiz are given below. But don’t look until you’ve tried to discover the answers by yourself.
⊗
SOLUTIONS
THAT’S ALL FOR NOW, FOLKS! http://www.wildi-theo.com
SI comment 16.doc
SEE YOU IN TWO WEEKS !
Copyright © 2005 Sperika Enterprises Ltd
All rights reserved





