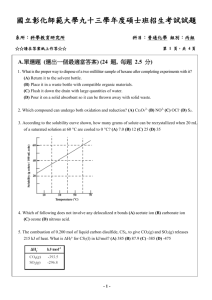
Redox stability in aqueous solution Let X be a species that is
advertisement

Redox stability in aqueous solution Let X be a species that is dissolved in water X + e → X- EoX Water can act as both a reducing agent: O2 + 4H+ + 4e → 2H2O Eo1 = 1.23V and an oxidising agent: 2H3O+ + 2e → 2H2O + H2 Eo2 = 0.00 V what are the permissible values of EoX for X to survive at a given pH? O2 + 4H+ + 4e → 2H2O Eo1 = 1.23V 0.0592 V E = E − log Q n E = 1.23 V − 0.0592 V x pH o 1 as we have seen before Similarly for 2H3O+ + 2e → 2H2O + H2 Eo2 = 0.00 V E = E o2 − 0.0592 V log Q n E = 0.00 V − 0.0592 V x pH Let’s look specifically at pH 7 O2 + 4H+ + 4e → 2H2O Eo1 = 1.23V E = 1.23 V − 0.0592 V x pH E pH7 = 1.23 -0.0592 x 7 = 0.82 V 2H3O+ + 2e → 2H2O + H2 Eo2 = 0.00 V E = 0.00 V − 0.0592 V x pH E pH7 = 0.00 -0.0592 x 7 = -0.41 V O2 + 4H+ + 4e → 2H2O Eo1 = 1.23V E pH7 = 0.82 V 2H3O+ + 2e → 2H2O + H2 Eo2 = 0.00 V E pH7 = -0.41 V X + e → X- EoX Suppose EoX < -0.41 V. For example, let EoX = -0.50 V 2X- → 2X + 2e 2H3O+ + 2e → H2 + 2H2O 2H3O+ + 2X- → 2X + 2H2O + H2 0.50 V -0.41 V 0.09 V The reaction is spontaneous and X- will not survive. It will be oxidised by water. O2 + 4H+ + 4e → 2H2O Eo1 = 1.23V E pH7 = 0.82 V 2H3O+ + 2e → 2H2O + H2 Eo2 = 0.00 V E pH7 = -0.41 V X + e → X- EoX Suppose EoX > 0.82 V. For example, let EoX = 0.90 V 4X + 4e → 4X2H2O → O2 + 4H+ + 4e 2H2O + 4X → 4X- + O2 + 4H+ 0.90 V -0.82 V 0.08 V The reaction is spontaneous and X will not survive. It will by reduced by water. Finally suppose -0.41 V < EoX < 0.82 V. For example, let EoX = 0.10 V 4X + 4e → 4X2H2O → O2 + 4H+ + 4e 2H2O + 4X → 4X- + O2 + 4H+ 0.10 V -0.82 V -0.72 V The reaction is not spontaneous. 2X- → 2X + 2e 2H3O+ + 2e → H2 + 2H2O 2H3O+ + 2X- → 2X + 2H2O + H2 The reaction is not spontaneous. -0.10 V -0.41 V -0.51 V O2 + 4H+ + 4e → 2H2O Eo1 = 1.23V E pH7 = 0.82 V 2H3O+ + 2e → 2H2O + H2 Eo2 = 0.00 V E pH7 = -0.41 V 0.82 V For a species to be stable in water, its redox potential must lie between these values at pH 7. This is sometimes referred to as the Electrochemical Window -0.41 V Recall, however, that the overpotential may make species with Eo values outside the electrochemical window kinetically stable – i.e., they can exist in solution because the kinetics of their reaction with water are slow By applying the Nernst equation, we can get the stability field of water at any pH. H2O oxidised above this line E = 1.23 V − 0.0592 V x pH E = 0.00 V − 0.0592 V x pH Figure 5.3 H2O reduced below this line Example Is Co(III) (a) thermodynamically and (b) kinetically stable in water at pH 0 and at pH 7? O2|H2O Co(III) + e → Co(II) At pH = 0 Eo = 1.92V 1.23 V 0.82 V pH 0 pH 7 0.00 V -0.41 V H+|H2 Example Is Co(III) (a) thermodynamically and (b) kinetically stable in water at pH 0 and at pH 7? O2|H2O Co(III) + e → Co(II) At pH = 7 Eo = 1.92V 1.23 V 0.82 V pH 0 pH 7 0.00 V -0.41 V H+|H2 Aluminium should react spontaneously with water at pH 7: Al3+ + 3e → Al O2|H2O Eo = -1.66 V H+|H2 2Al → 2Al3+ + 6e 6H+ + 6e → 3H2 Eo = 1.66 V EpH7 = -0.41 2Al + 6H+ → 2Al3+ + 3H2 Eo = 1.25 V pH 7 0.82 V -0.41 V It doesn’t because the surface is passivated by the formation of a tough coating of Al2O3 which sticks tightly to the bulk Al metal and protects it. This can be enhanced by anodising the metal. The metal is made the anode in an electrolytical cell and oxidised to form the protective film of the oxide. Nitric acid can be used to passivate some metals such as stainless steels. Some couples have very positive potentials but the species can still exist is water. Examples include: O2|H2O pH 7 Cr2O72-|Cr3+ Eo = 1.38 V 0.82 V 2+ o MnO4 |Mn E = 1.51 V Hence at pH 7 the Ecell for oxidation of water to produce O2 is 0.56 V and 0.69 V, respectively. H+|H2 Reason why Cr2O72- and MnO4- can still exist: Requires a 6e and a 5e transfer, respectively. Multiple electron transfer reactions are usually very slow. These species are under kinetic control -0.41 V Natural waters potential controlled by amount of dissolved O2 and by the presence of reducing couples from organic matter Fig.5.12 pH controlled by CO2/H2CO3/HCO3-/CO32- equilibrium Disproportionation reactions Since Cu+|Cu Cu2+|Cu+ Eo = 0.52V Eo = 0.16 V Both potentials lie within the stability field of water Cu+ ions neither oxidise nor reduce water But Cu+ ions in solution are unstable because they can undergo disproportionation 2Cu+ → Cu2+ + Cu(s) A disproportionation reaction is one where the oxidation state of an element is simultaneously increased and decreased Disproportionation occurs because: Cu+ + e → Cu Cu+ → Cu2+ + e Eo = 0.52 Eo = -0.16 2Cu+ → Cu + Cu2+ Eo = 0.36 V Example In a comproportionation reaction two species of an element in two different oxidation states form a product in which the element is in an intermediate oxidation state Ag2+ + Ag → 2Ag+ Eo = 1.18 V Example Show that the equilibrium constant lies far to the right for the following comproportionation reaction at 25 °C: Ag2+ + Ag → 2Ag+ Eo = 1.18 V Representing potential data Latimer diagrams summarise the standard potential (in V) between species of an element. +1.20 ClO4+7 +1.18 +1.65 ClO3- HClO2 +5 +3 +1.67 HClO +1 +1.36 Cl2 Cl- 0 -1 in acid So we can immediately write down the half reaction and the potential connecting any two species. For example, ClO3- and HClO2 ClO3- HClO2 - Balance O by adding H2O ClO3- HClO2 + H2O - In acid, balance H by adding H+. (In base, add H2O to the side of the equation requiring H, and OH- to the other side.) ClO3- + 3H+ HClO2 + H2O - Balance charges by adding e ClO3- + 3H+ + 2e HClO2 + H2O Eo = +1.18V +1.20 ClO4+7 +1.18 +1.65 ClO3- HClO2 +5 +3 +1.67 HClO +1.36 Cl2 Cl- 0 -1 +1 in acid Suppose we are interested in the potential between two non-adjacent couples. Cannot just add their standard potentials! ∆rG°overall = ∆rG°individual steps (First Law of Thermodynamics) i.e. ∆rG°(a+b ) = ∆rG°(a ) + ∆rG°(b ) -noverall FEooverall = -n1FEo1 + -n2FEo2 + -n3FEo3 +1.20 ClO4+7 +1.18 +1.65 ClO3- HClO2 +5 +3 +1.67 HClO +1 +1.36 Cl2 Cl- 0 -1 in acid +1.20 ClO4+7 +1.18 +1.65 ClO3- HClO2 +5 +3 +1.67 HClO +1 +1.36 Cl2 Cl- 0 -1 and this refers to the reaction HClO2 + 3H+ + 4e Cl- + 2H2O in acid